
In August 2017, food safety authorities in the Member States of the European Union (EU) and the food industry have implemented significant monitoring of eggs for residues of fipronil, which is being conducted to ensure that the recall measures are protecting consumers. There is also interest in egg products, meat, and organs from laying poultry. In order to monitor fipronil abuse and ensure the safety of such foods, a simple, sensitive, reliable, and validated method for determining residues of fipronil in chicken egg is needed.
The default EU MRL for fipronil in eggs is set at 0.005 mg/kg with a residue definition of the sum of the parent fipronil and the metabolite fipronil sulfone, expressed as fipronil.2 Fipronil and fipronil sulfone can be determined by either LC-MS/MS or GC-MS(/MS) after a generic extraction such as QuEChERS , followed by clean up with SPE, either in dispersive (dSPE), or in pass-through modes. When analyzing these compounds using LC-MS/MS with electrospray in negative ion mode, consideration should be given to the impact of matrix effects from co-eluting co-extractives that can suppress the signal, reducing the sensitivity, accuracy, and robustness of the method. A balance must be struck between providing a rapid analytical method and the need for accurate quantification and robustness.
In this application note, we report the results of a validation of a modified QuEChERS method for the determination of fipronil and its metabolite fipronil sulfone, in eggs by liquid chromatography-tandem quadrupole mass spectrometry, which meets the SANTE criteria (SANTE/11945/2015).
A robust, cost-effective method for the determination of fipronil and fipronil sulfone in eggs, that meets requirements for both official control and food business operators’ due diligence testing, at concentrations significantly lower than the EU MRL.
Fipronil is an insecticide used to protect seeds from insects, for professional pest control to combat infestation of insects such as cockroaches, as well as in veterinary medicine to protect dogs and cats from fleas, mites, and ticks. Fipronil is highly toxic and it is not authorized for use as a veterinary medicine, biocide, or pesticide around food producing animals. Hence it should never have found its way into a chicken coop.
At the center of the recent food safety concern throughout Europe, fipronil has been found in eggs at concentrations above the maximum residue level (MRL).1 With ongoing police investigations, product recalls, and destruction of many millions of eggs, increased analytical testing has ensued to ensure consumer safety throughout Europe and as far afield as Hong Kong.
Food safety authorities in the Member States of the European Union (EU) and the food industry have implemented significant monitoring of eggs for residues of fipronil, which is being conducted to ensure that the recall measures are protecting consumers. There is also interest in egg products, meat, and organs from laying poultry. In order to monitor fipronil abuse and ensure the safety of such foods, a simple, sensitive, reliable, and validated method for determining residues of fipronil in chicken egg is needed.
The default EU MRL for fipronil in eggs is set at 0.005 mg/kg with a residue definition of the sum of the parent fipronil and the metabolite fipronil sulfone, expressed as fipronil.2 Fipronil and fipronil sulfone can be determined by either LC-MS/MS or GC-MS(/MS) after a generic extraction such as QuEChERS , followed by clean up with SPE, either in dispersive (dSPE) or in pass-through modes. When analyzing these compounds using LC-MS/MS with electrospray, consideration should be given to the impact of matrix effects from co-eluting co-extractives that can suppress the signal, reducing the sensitivity, accuracy, and robustness of the method. A balance must be struck between providing a rapid analytical method and the need for accurate quantification and robustness.
In this application note, we report the results of a validation of a modified QuEChERS method for the determination of fipronil and its metabolite fipronil sulfone in eggs by liquid chromatography-tandem quadrupole mass spectrometry, which meets the SANTE criteria (SANTE/11945/2015).3
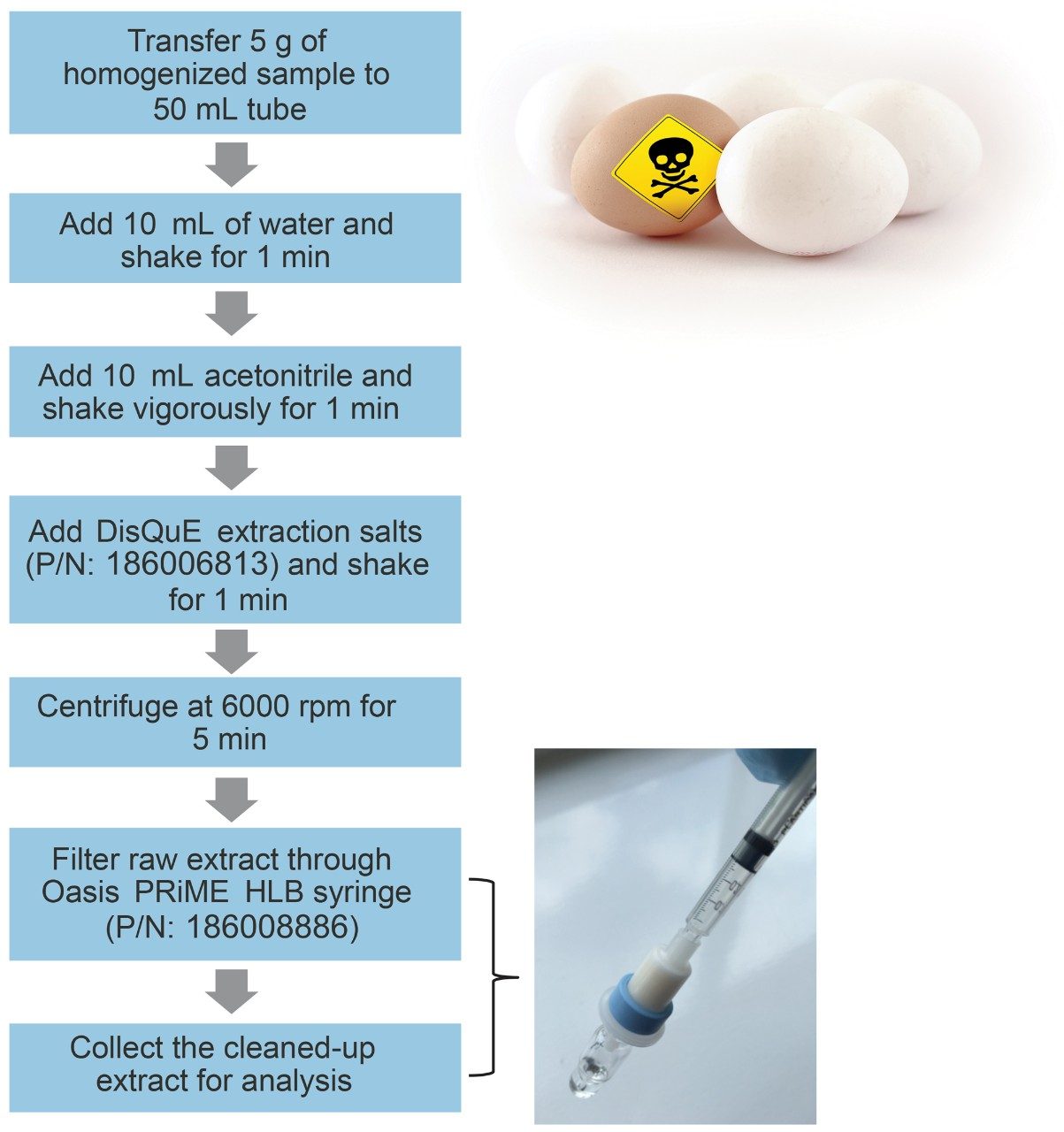
Eggs were purchased from a local shop and extracted using a modified QuEChERS method.4 The sample preparation workflow employed in this method is summarized in Figure 1.
The performance of the method was assessed using SANTE guidelines. To assess accuracy and precision of the method, test portions of eggs were spiked at two concentrations; 0.002 mg/kg and 0.02 mg/kg (n=5). Solutions of standards were prepared over the range 0.0005 to 0.05 mg/kg (0.5 to 50 ppb) in solvent and in egg extract (matrix matched), to determine the concentration of fipronil and fipronil sulfone in the spikes (using bracketed calibration) and to evaluate matrix effects.
|
UPLC system: |
ACQUITY UPLC I-Class with FTN Sample Manager |
|
Column: |
ACQUITY UPLC HSS T3, 1.8 μm, 2.1 × 100 mm |
|
Mobile phase A: |
2 mM Ammonium acetate (aq.) |
|
Mobile phase B: |
Acetonitrile (LC/MS grade) |
|
Flow rate: |
0.4 mL/min |
|
Injection volume: |
3 μL |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Runtime: |
8.5 min |
|
Time |
%A |
%B |
Curve |
|---|---|---|---|
|
Initial |
95.0 |
5.0 |
Initial |
|
0.5 |
95.0 |
5.0 |
6.0 |
|
5.0 |
2.0 |
98.0 |
6.0 |
|
7.0 |
2.0 |
98.0 |
6.0 |
|
7.5 |
95.0 |
5.0 |
6.0 |
|
8.5 |
95.0 |
5.0 |
1.0 |
|
MS system: |
Xevo TQ-XS |
|
Source: |
Electrospray |
|
Ionization mode: |
ESI Capillary |
|
Voltage: |
2.0 kV |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas flow: |
800 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/Hr |
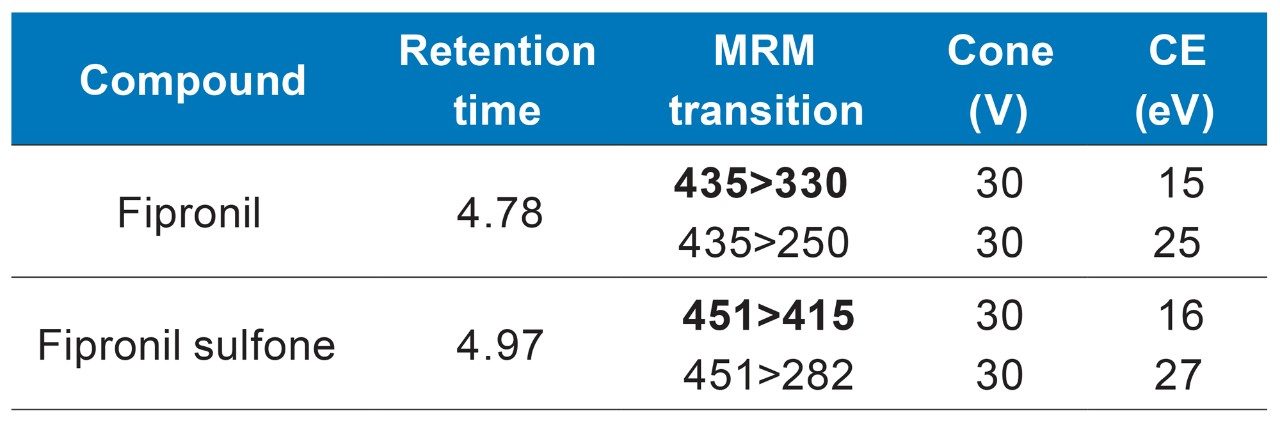
The two MRM transitions that showed the best selectivity were used for each of the analytes. Data were acquired using MassLynx MS Software (v4.2) and processed using TargetLynx XS Application Manager. The optimum dwell time was set automatically using the autodwell function based on 4 s wide peaks and 12 data points per peak.
Within-laboratory method validation should be conducted to provide evidence that a method is fit for the purpose for which it is to be used. To meet the requirements of the SANTE guidelines this method has been tested to assess sensitivity, mean recovery (as a measure of trueness or bias), precision (as repeatability RSDr) and the method limit of quantification (LOQ).
A minimum of five replicates were required (to check recovery and precision) both at the targeted LOQ of the method and at least one other higher level. The lower concentration, the targeted LOQ, was set to 0.002 mg/kg to accommodate the residue definition for fipronil in eggs; the sum of fipronil and fipronil sulfone, expressed as fipronil. The higher concentration was set at 0.020 mg/kg, 10x the targeted LOQ.
Validation of the method demonstrated excellent performance for the identification and quantification of fipronil and fipronil sulfone in egg. These results are summarized in Table 2, showing that all of the relevant criteria set out in SANTE guidelines have been met. These analytical criteria and subsequent results are discussed, below, in more detail.
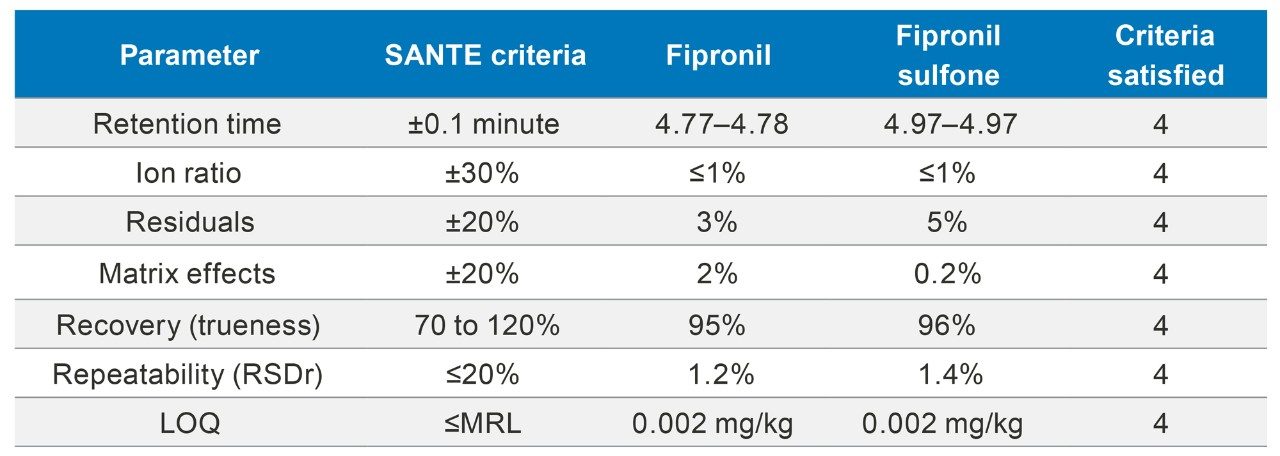
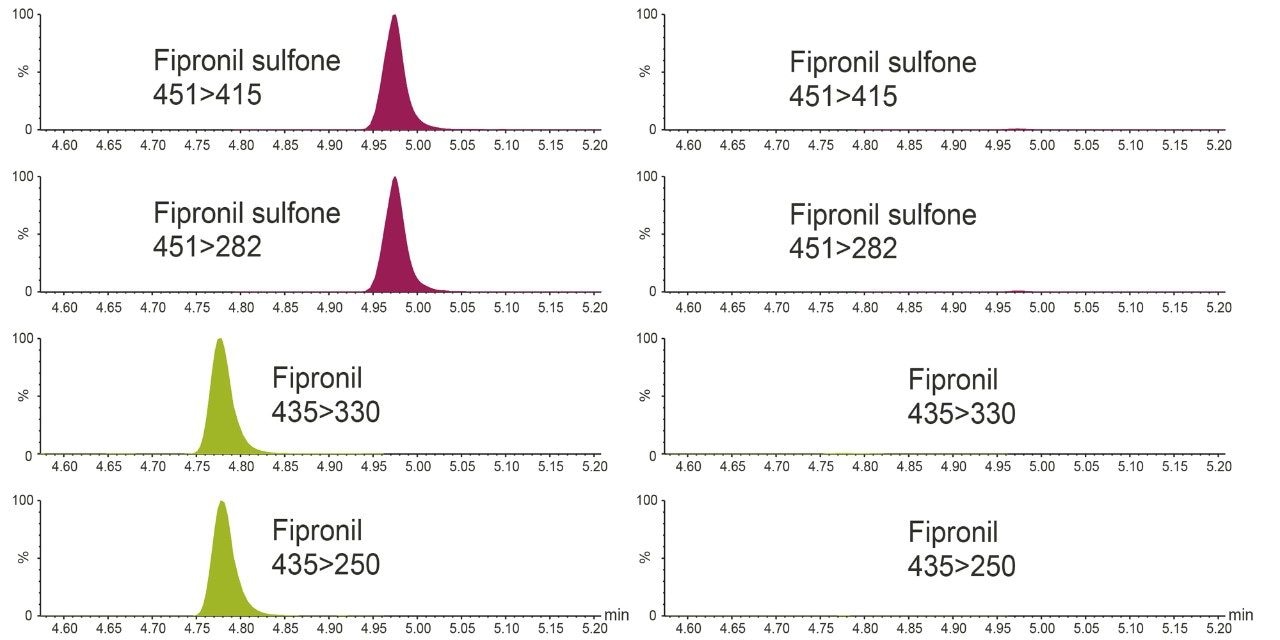
Figure 2 shows the detection of fipronil and fipronil sulfone in a matrix-matched standard in eggs at 0.0005 mg/kg (0.5 ppb). This demonstrates the excellent sensitivity and selectivity of the method and its suitability for checking compliance with the EU MRL of 0.005 mg/kg as well as the potential for screening and quantification at much lower concentrations. Residues were detected in the egg sample chosen as the blank but at very low concentrations (estimated as ca. 0.02 ppb).
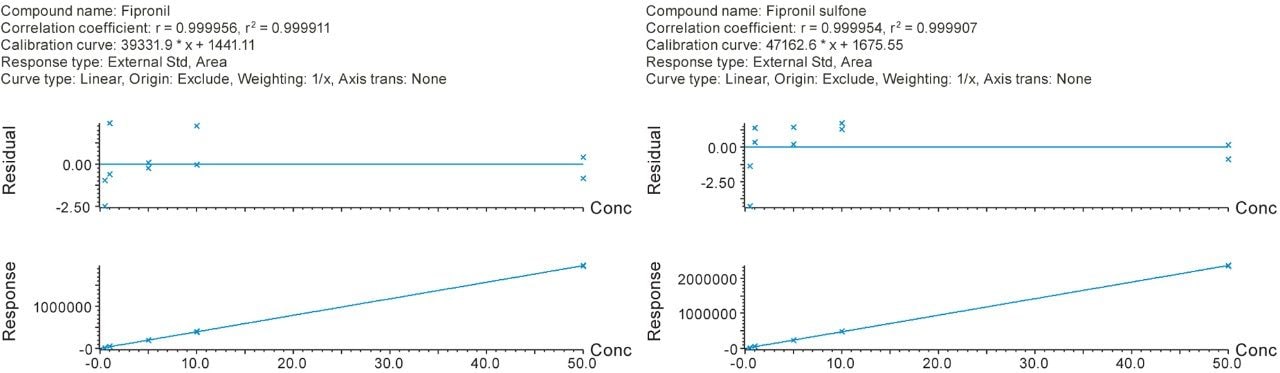
The linearity of response for fipronil and fipronil sulfone in egg matrix was evaluated using bracketed calibration over a suitable concentration range; 0.0005 to 0.05 mg/kg (0.5 to 50 ppb) as shown in Figure 3. The coefficients of determination and the residuals were satisfactory (r2>0.999 and residuals <5%). Comparison of the slope of the matrix-matched calibration curve to the one prepared in solvent demonstrated that the use of Oasis PRiME HLB successfully removed any co-eluting co-extractives, as the matrix effects were observed to be minimal (ca. 2%).
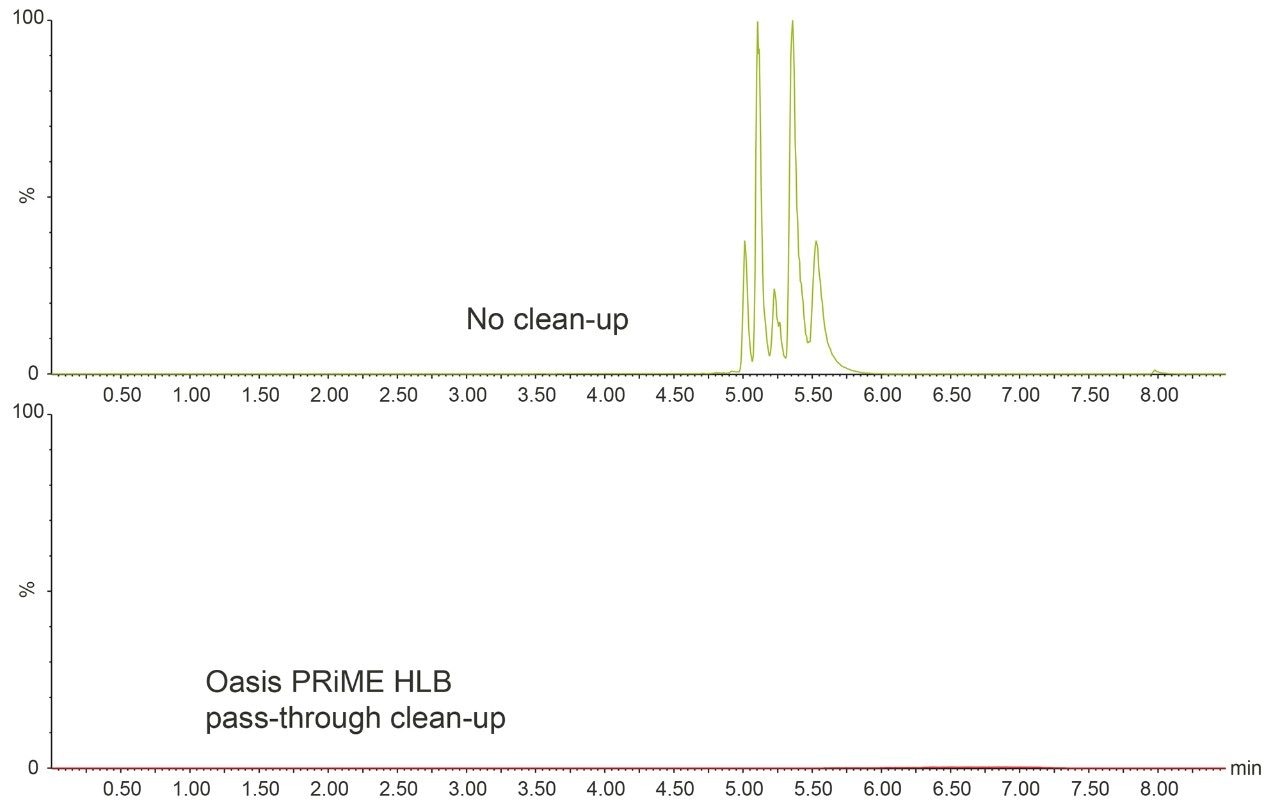
When the Oasis PRiME HLB cleanup step was included, at least 95% of phospholipids were removed, as one can see by comparing the phospholipid response from analysis of egg extracts, before and after cleanup (Figure 4). As well as contributing to matrix effects, such endogenous material builds up in the LC-MS/MS system. Cleanup minimizes such contamination and the frequency of manual intervention and maintenance is dramatically decreased.
To assess the accuracy and precision of the method, test portions of a blank egg sample were spiked at two concentrations, each with five replicates. Mean recovery and repeatability (RSDr) for fipronil and fipronil sulfone was 95% (1.2% RSD) and 96% (1.4% RSD), respectively.
Table 3 shows the concentrations of fipronil and fipronil sulfone determined in each spike. Ion ratio and retention times agreed well with the reference values derived from the matrix-matched standards and were well within the required tolerances.
720006094, August 2017