Polysorbates are non-ionic surfactants widely used as excipients in food and pharmaceutical products. As their quality and purity relate to the safety of the drug products and patients’ health, quality assurance and control using suitable test methods should be implemented. The U.S. Pharmacopoeia specifies a gas chromatography (GC) with flame ionization detector (FID) procedure for polysorbate 20 based on fatty acids composition by conversion of methylated acids to free fatty acids (USP–NF 2021 Issue 1). In this work, an HPLC method coupled with mass spectrometry (MS) for the analysis of polysorbate 20 pharmaceutical raw material is presented. The new method offers a fast and direct analysis by measuring fatty acids composition using mass detection, eliminating the need for a complex sample preparation procedure.
Polysorbates are non-ionic surfactants often referred by their trade name as Tweens.1–3 They are widely used as emulsifiers, stabilizers, wetting agents, solubilizers, and dispersants in the manufacturing of pharmaceutical drug products.3
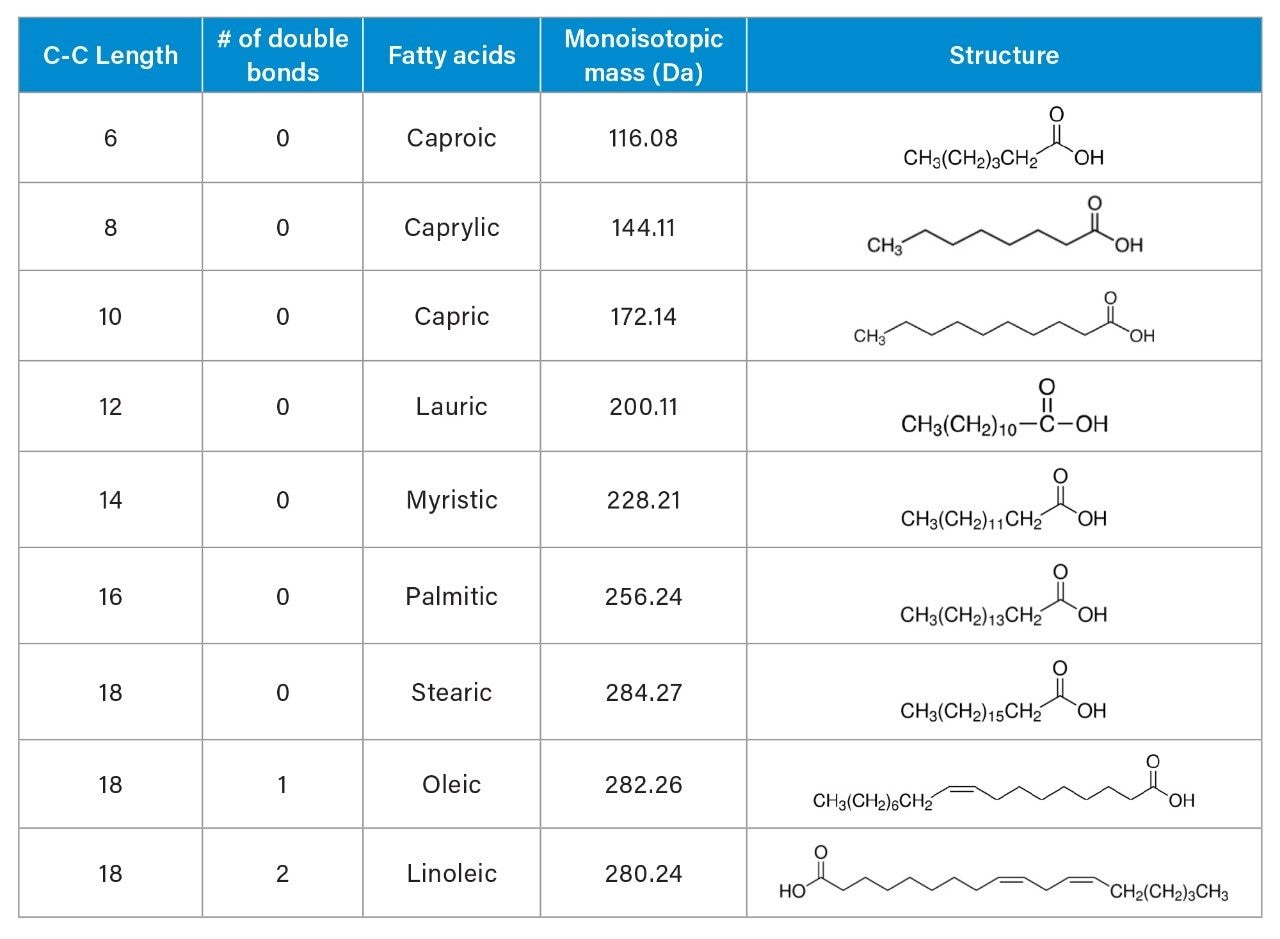
Polysorbates are complex mixtures of multiple components, composed of polyoxyethylated sorbitan monoesters of saturated and/or unsaturated fatty acids.1 The differences between types of polysorbates are fatty acid chain side and the degree of esterification. Due to the complex and heterogenous nature, the polysorbates analysis can be a challenging task. Additionally, polysorbates lack strong chromophore required for UV detection. A variety of analytical techniques have been developed and applied to analyze and characterize major polysorbates subspecies, such as liquid chromatography (LC) combined with charged aerosol detector (CAD), evaporative light scattering detector (ELSD), and mass spectrometry.1–3 Other techniques utilize gas chromatography (GC) with mass detection and nuclear magnetic resonance (NMR) technology.2 Several reported methods allow quantification of polysorbates as a single peak, but do not provide specificity for all fatty acids .2 Another method describes the quantification of free fatty acids in biopharmaceutical formulations containing polysorbate 204.
Polysorbate 20 is widely used the in pharmaceutical products to stabilize emulsions and suspensions and as a lubricant in ophthalmic solutions.5 The U.S. Pharmacopeia recommends a gas chromatography method with flame ionization detector (FID) for polysorbate 20 analysis based on fatty acids composition by conversion of methylated acids to free fatty acids.6 This procedure requires hydrolysis and derivatization of the polysorbate to free fatty acids. This is a complex and time-consuming procedure, not ideal for routine testing within QC laboratory.
In this work, we present a fast HPLC method with mass detection for the analysis of polysorbate 20 pharmaceutical raw material based on fatty acids composition (Table 1). This method enables quick and accurate analysis of fatty acids by direct injection, eliminating a complex sample preparation procedure and the need for gas chromatography instrumentation.
Fatty acids, polysorbate 20, and mass spectrometry grade solvents were purchased from Sigma.
Stock standard solutions of fatty acids were prepared in ethanol at 1 mg/mL concentrations. Stock standard solutions were diluted with a mixture of water/ethanol (50:50, v/v) to 20 µg/mL concentration.
Polysorbate 20 test samples were hydrolyzed with 1 M potassium hydroxide in water to release fatty acids. The test samples prepared in 1 M potassium hydroxide at 1.5 mg/mL were incubated for 6 hours at 40 °C. Solutions were then neutralized with equal volume of 1 M formic acid, diluted with mixture of water/ethanol (50:50, v/v) to 0.1 mg/mL, and filtered through PVDF syringe filters prior analysis.
|
LC Conditions |
|
|
LC system: |
Arc HPLC System, Column heater/cooler with passive pre-heater, ACQUITY QDa Detector, isocratic solvent manager (ISM) |
|
Vials: |
LCMS Maximum Recovery 2 mL volume (p/n: 600000670CV) |
|
Column(s): |
XBridge BEH C18, 4.6 x 100 mm, 3.5 µm (p/n: 186003033) |
|
Column temp.: |
60 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
25 µL |
|
Flow rate: |
2.0 mL/min |
|
Mobile phase: |
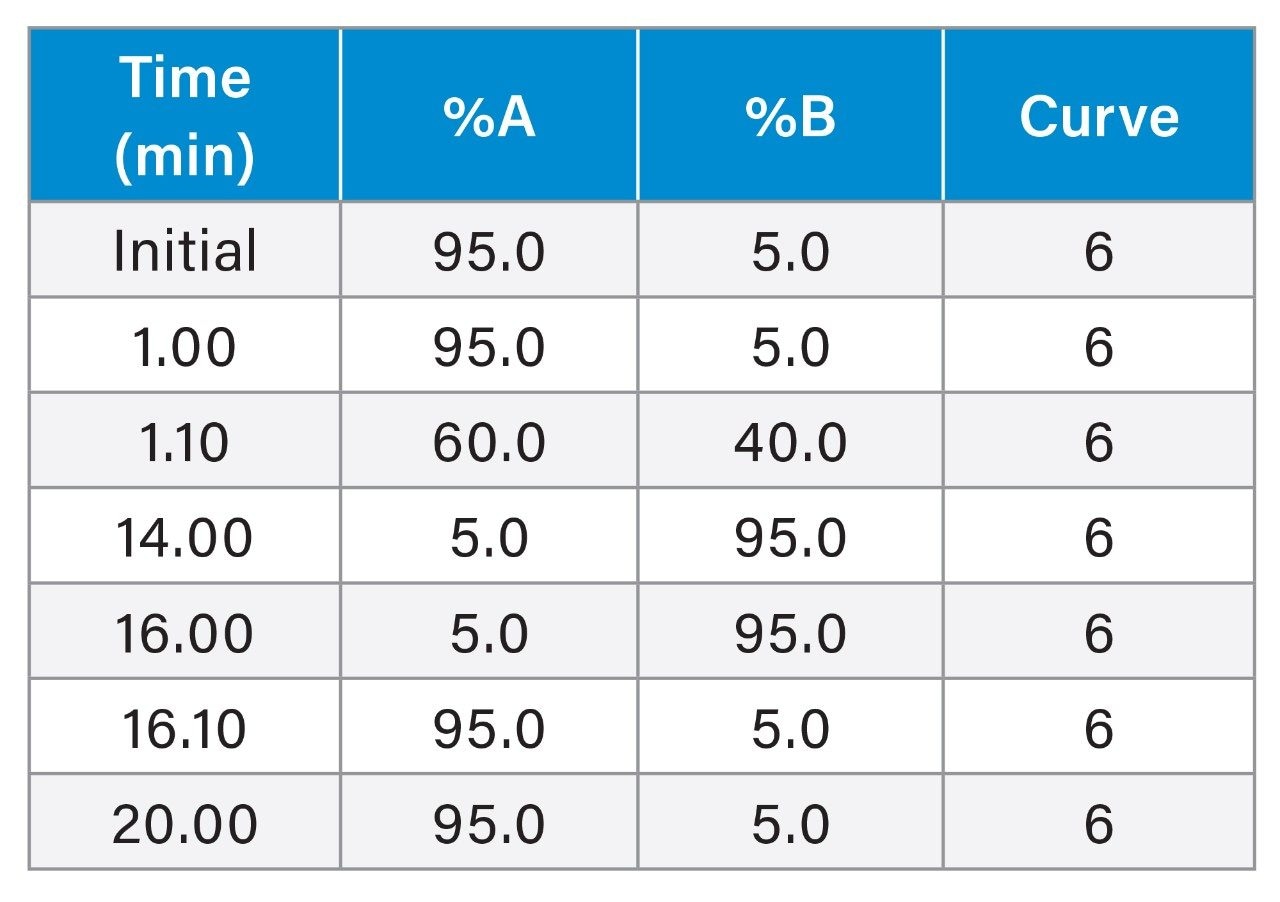
Solvent A: 10 mM Ammonium acetate in water Solvent B: Acetonitrile |
|
Wash solvents: |
Purge/Sample wash: 60:40 Water/acetonitrile Seal wash: 90:10 Water/acetonitrile |
|
MS system: |
ACQUITY QDa Detector, Performance Option |
|
Ionization mode: |
ESI- |
|
Acquisition range: |
75–350 m/z |
|
Single ion recording (SIR): |
115.1, 143.1, 171.2, 199.2, 227.3, 279.3, 255.3, 281.3, 283.3 m/z |
|
Probe temperature: |
600 °C |
|
Capillary voltage: |
0.5 kV |
|
Cone voltage: |
10 V |
|
Makeup solvent: |
50:50 Water/acetonitrile with 1 mM ammonium acetate |
|
Flow rate: |
0.2 mL/min, with 10:1 split and dilute ratio |
|
Chromatography software: |
Empower 3 FR4 SR2 |
|
The method in this work is based on the hydrolysis of polysorbate 20 to release fatty acids followed by reverse-phase separation and determination of fatty acids composition by mass detection.
The saturated fatty acids lack chromophores (or double bonds), thus are not detectable by UV (Table 1). They cannot be directly detected by UV, but produce a robust signal on the ACQUITY QDa Detector. The MS total ion chromatogram (TIC) shows all peaks detected across the mass range of m/z 75–350 in ESI- mode (Figure 1A). The mass spectra data from the ACQUITY QDa Detector enables quick identification of the fatty acids. The single ion recording (SIR) mode, which determines the intensity for a single ion of interest (Figure 1B), enhances signal and simplifies analysis for targeted compounds. For quantitative analysis, fatty acids were measured using a single ion recording (SIR) mode.
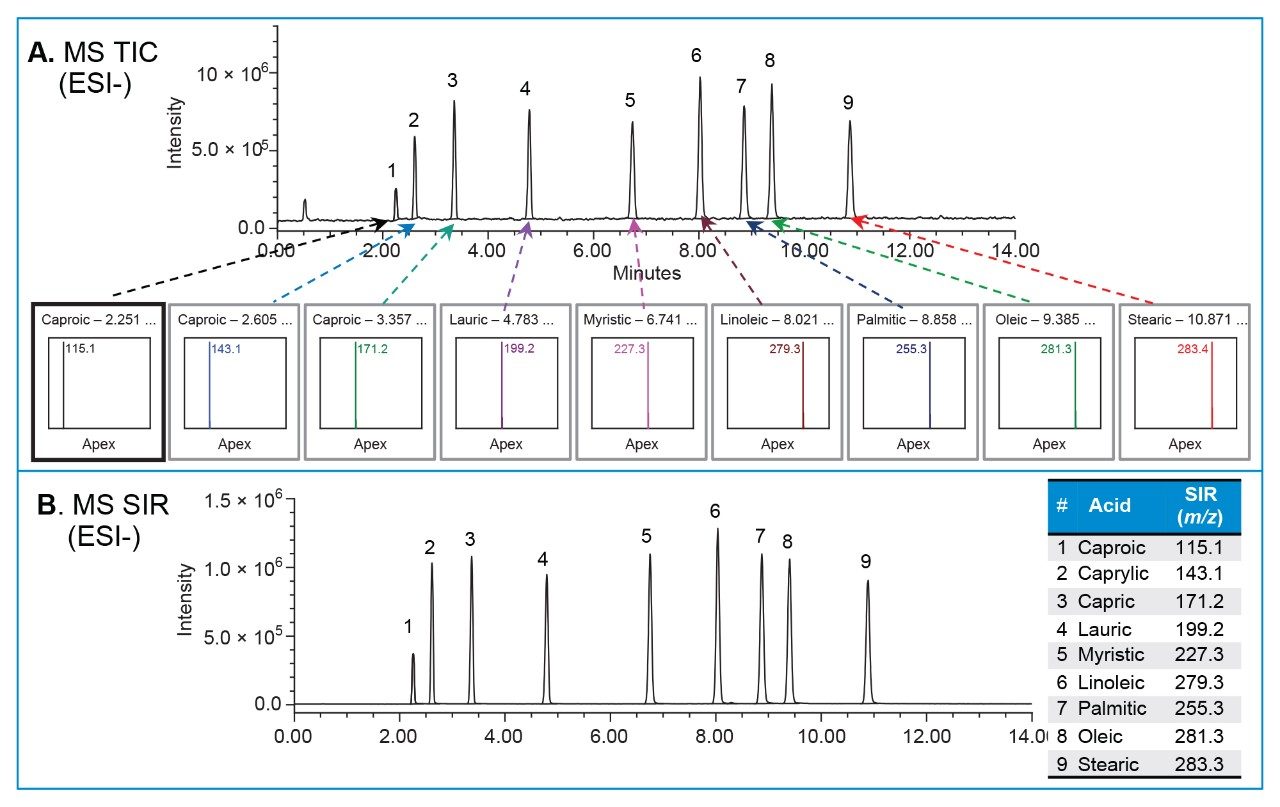
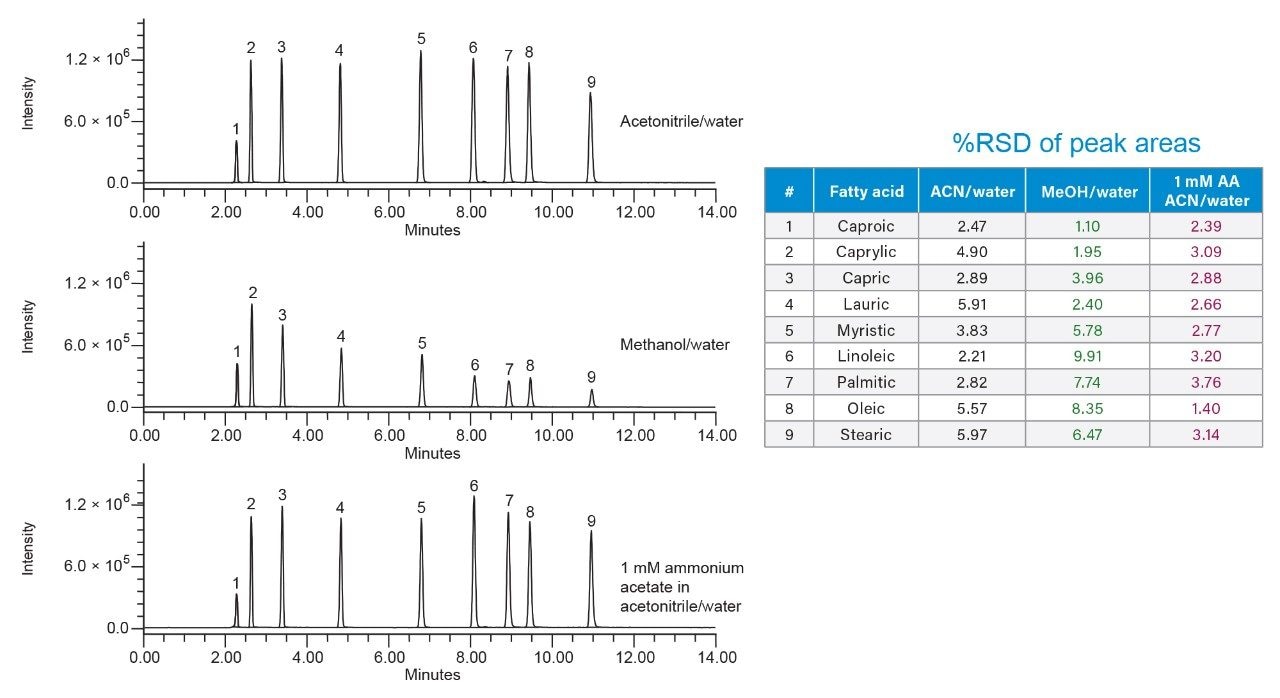
The isocratic solvent manager (ISM)7 was used to split and dilute the flow entering the ACQUITY QDa Detector. The ISM make-up (dilution) solvent was added post-column and mixed with the flow entering the source. Various ISM makeup solvents containing acetonitrile, methanol and ammonium acetate buffer were screened during the study to enhance the MS signal, while ensuring acceptable repeatability of replicate injections. For example, makeup solvent with acetonitrile and ammonium acetate (Figure 2) provided robust signal for fatty acids with acceptable repeatability of the replicate injections (n = 5). The %RSD of peak areas with 1 mM ammonium acetate makeup solvent were lower compared to other makeup solvents screened during the study.
Performance of the method was measured by evaluating system suitability of 6 replicate injections of the 20 µg/mL fatty acid standard (Figure 3) following the specifications listed in the USP general chapter <621>, Chromatography.8 The method successfully separated all fatty acids with a USP resolution (Rs) ≥4.2 between the peaks. Repeatability of the retention times and peak areas ranged from 0.13 to 0.62 and 1.29 to 4.19 %RSD, respectively.
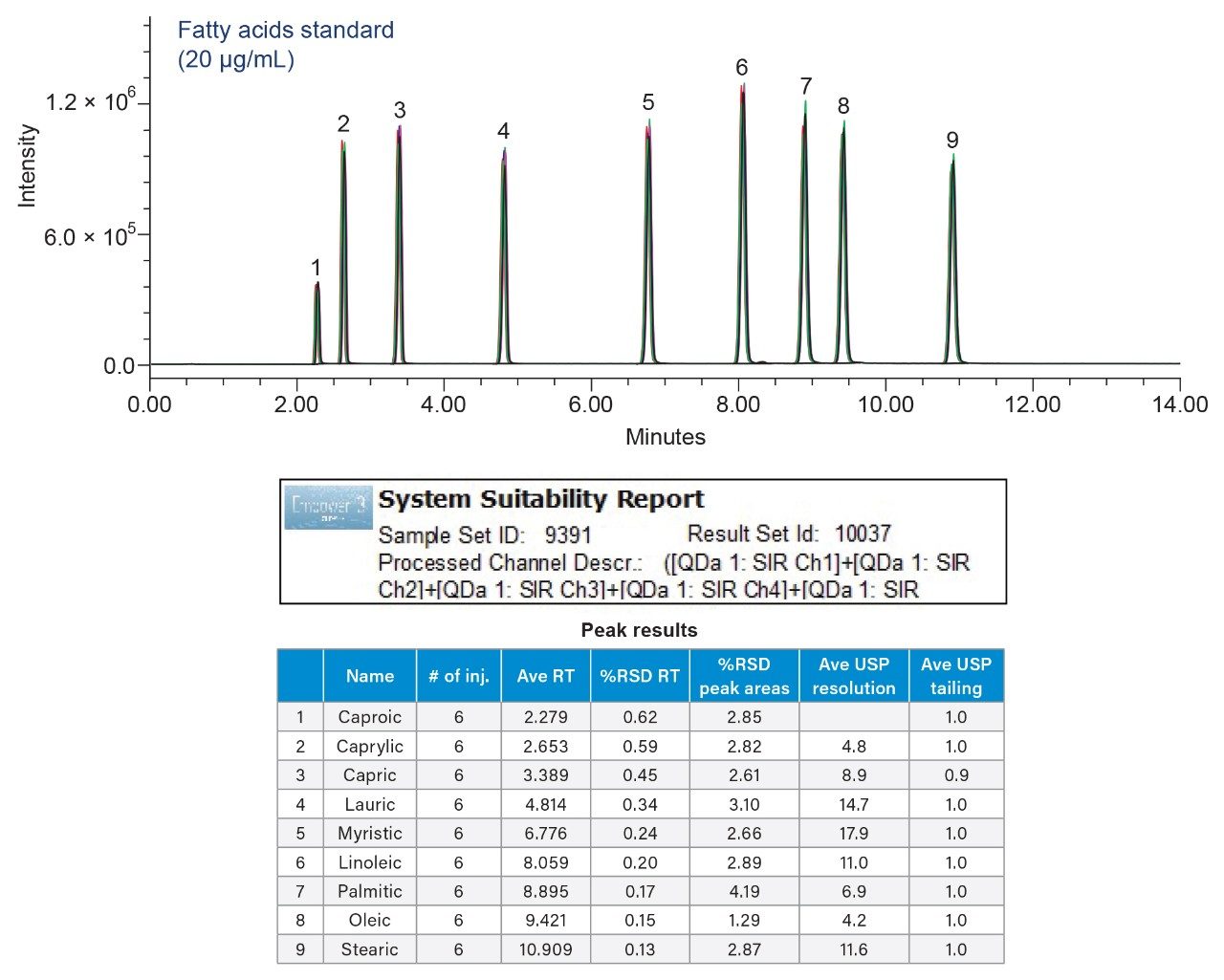
The percent (%) of each fatty acid in the polysorbate 20 samples was calculated using Empower Software by comparing peak area of each fatty acid to the total area of all fatty acids in the chromatographic injections. The calculations were performed following the USP monograph for polysorbate 20.6 The results for composition of fatty acids in the polysorbate 20 sample solutions met the USP criteria (Figure 4).
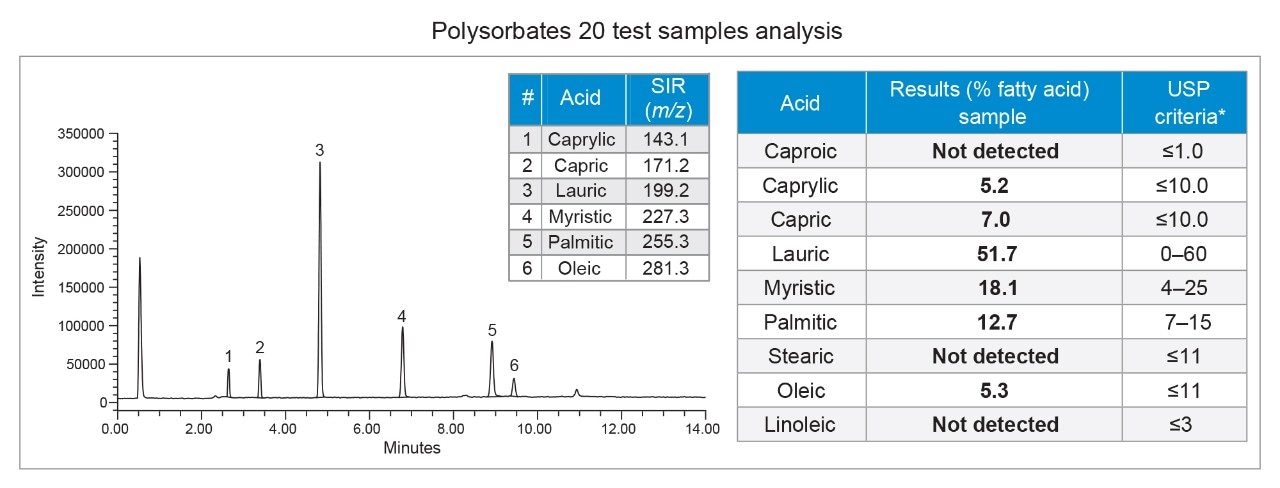
The developed HPLC-MS method allows fast determination of fatty acids composition in polysorbate 20 and eliminates the need for a complex hydrolysis and derivatization procedure of the test samples. The mass spectral data enables accurate and quick identification of analytes by mass detection. The new method offers accurate quality and purity assessment of polysorbate 20 pharmaceutical raw material, while improving the confidence associated with sample component confirmation.
Overall, the Arc HPLC is a modern instrument that delivers powerful performance, high injection precision, low carryover, and high backpressure tolerance. The ACQUITY QDa Detector is a robust and simple-to-use mass detector that provides accurate and reliable results. These technologies can be easily adapted for routine testing of pharmaceutical raw materials in the QC Laboratory.
720007336, Revised November 2021