This application note describes use of two structurally similar drugs, imipramine and amitriptyline to simulate a difficult mixture of an API and a closely eluting impurity present at low levels.
The LC-MS analysis of trace level impurities is an important capability in numerous fields. Some chemical processes have acceptance criteria focused specifically on the presence and amount of impurities. This is especially true in the pharmaceutical industry. In such analyses, the intense and often broad main component(s) (e.g., API) can obscure adjacent impurity peaks, making quantitation difficult and irreproducible. Furthermore, a significant amount of pharmaceutical compounds are basic in nature which present challenges in the chromatographic separation due to poor peak shape at low pH.
This application note describes use of two structurally similar drugs, imipramine and amitriptyline to simulate a difficult mixture of an API and a closely eluting impurity present at low levels. These compounds present a common analytical challenge. Their basic nature leads to poor peak shape in frequently employed formic acid mobile phase conditions. Such phenomena are mitigated with the CORTECS C18+ columns due to the surface charge present. This technology and the solid-core particles afford superior peak shape and higher MS signal. The result is more accurate and reliable characterization of the impurity peaks using HPLC-MS and UPLC-MS instruments.
|
Columns: |
CORTECS C18+, 2.7 μm, 3.0 x 50 mm (p/n 186007400) Competitor solid-core C18, 2.6 μm, 3.0 x 50 mm |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Flow rate: |
0.8 mL/min |
|
Column temp.: |
30 °C |
|
Detection (UV): |
254 nm |
|
Injection volume: |
10.0 μL |
|
Method: |
Start at 25% B, linear gradient to 35% B in 3.0 minutes. Return to 25% B in 0.1 minutes. Hold for 1 minute. |
|
System: |
ACQUITY TQD Mass Spectrometer |
|
Ionization mode: |
ESI+ Mode |
|
Capillary voltage: |
3.8 kV |
|
Cone voltage: |
30 V |
|
Source temp.: |
120 °C |
|
Desolvation temp: |
450 °C |
|
Cone gas flow: |
30 L/hr |
|
Desolvation gas flow: |
1000 L/hr |
|
Method: |
Selected Ion Recording (SIR) mode at 278.4 amu |
|
Data Management: |
Empower 3 CDS |
An aqueous solution of imipramine (0.5 mg/mL, simulated API) and amitriptyline (0.5 μg/mL, simulated impurity) was prepared and placed into an LCMS Certified Max Recovery Vial (p/n 600000749CV) for analysis.
Impurity analysis is an important testing method, especially in pharmaceutical manufacturing. Some validated USP methods require impurity testing as part of the overall sample analysis. Characterization and quantitation of impurities can be problematic in formulated samples as there are often other compounds besides the active pharmaceutical ingredient (API) and related impurities in the separation. Achieving a good separation between the API and the impurities is important both for characterization and quantitation of the components. Sharp, symmetrical peaks with good separation allows for more accurate analysis. CORTECS Columns allow rapid separation of compounds while displaying high efficiency leading to better separations. An example of this is the separation of imipramine and amitriptyline using a CORTECS C18+, 2.7 μm Column compared to a competitor solid-core C18 column.
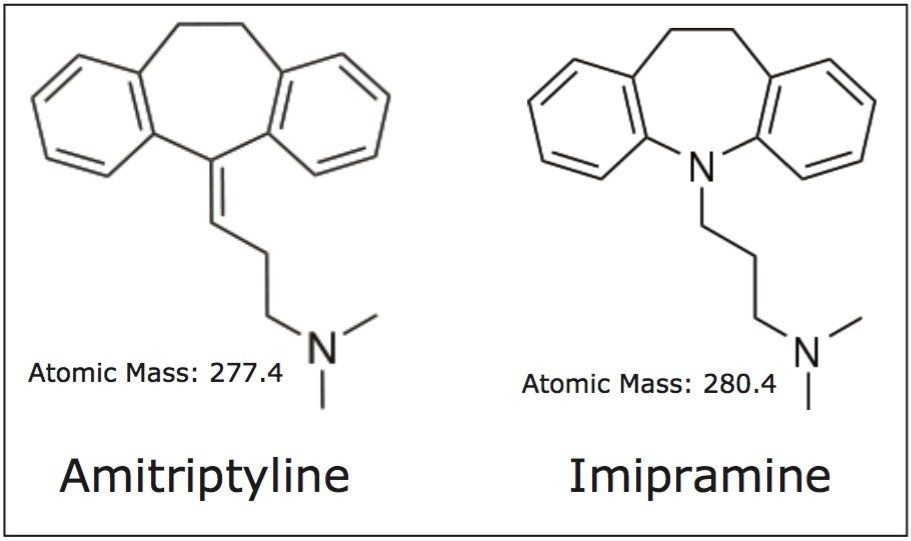
Amitriptyline and imipramine are basic compounds that belong to a group of drugs called tricyclic antidepressants. The structure of both compounds is shown in Figure 1. These compounds are structurally similar and both contain a tertiary amine on the end of a short carbon chain. These tertiary amines give the compounds their basic characteristics, which can present problems in reversed-phase LC analysis. Often, basic compounds can have poor peak shapes in low ionic strength acidic modifiers such as formic acid. However, the CORTECS C18+, 2.7 μm Column provides better peak shapes for bases using these conditions than a typical C18 column, due to the slightly positive surface charge on the C18+ stationary phase particles. This improvement is comparable to separations using TFA-modified mobile phases, but avoids the ion suppressing effect of TFA.
For this analysis, an ACQUITY UPLC System was configured with a PDA and an ACQUITY TQD for UV and MS detection. A CORTECS C18+, 2.7 μm Column and a competitor solid-core C18 column were compared for this separation. A sample containing 0.5 mg/mL Imipramine, and 0.5 μg/mL amitriptyline (0.1% the level of Imipramine) was prepared in water and 10 μL of that solution was injected onto each column. The UV chromatogram data were collected at 254 nm. The separations on both the CORTECS C18+, 2.7 μm Column and the competitor solid-core C18, 2.6 μm column are shown in Figure 2.
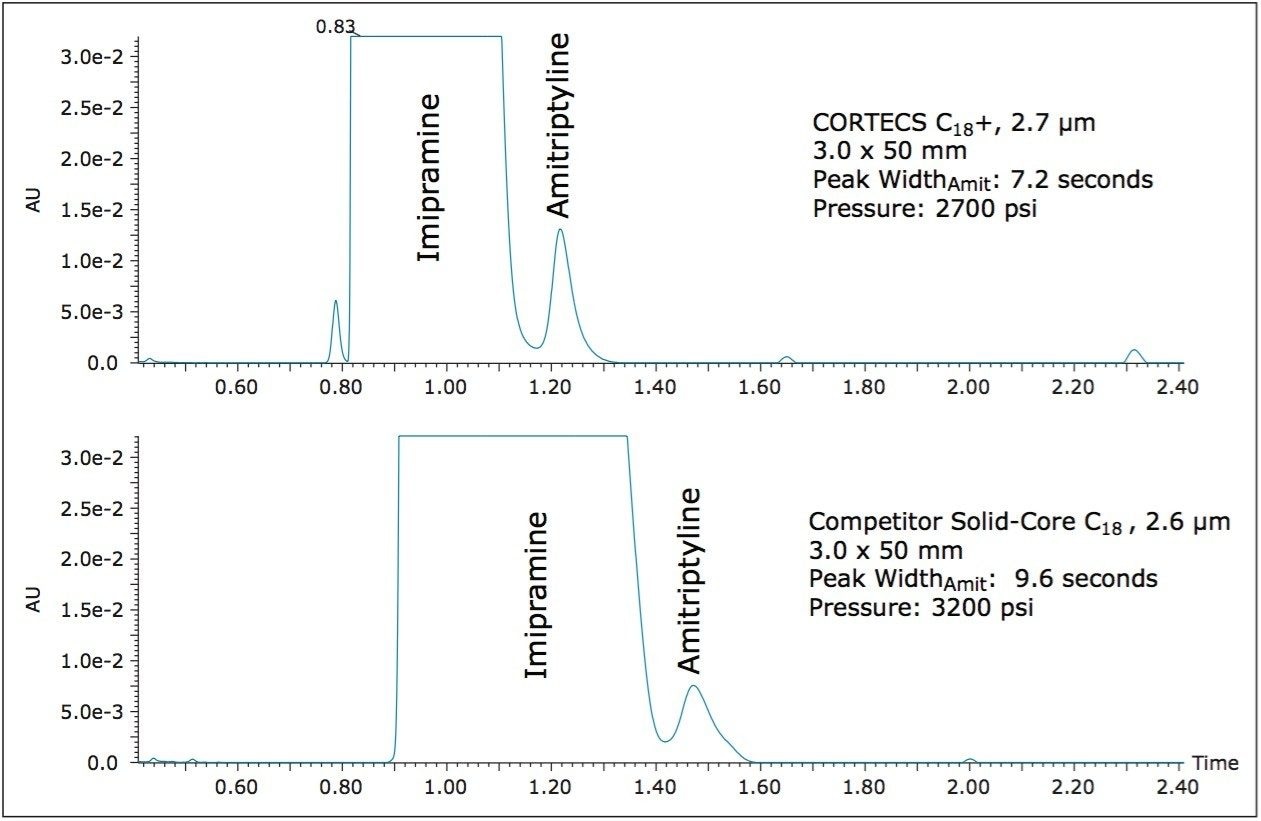
As the UV data show, the CORTECS C18+, 2.7 μm Column yields a narrower peak for the simulated impurity, amitriptyline, with increased intensity in UV as well. Additionally the simulated API peak, Imipramine, is much narrower on the CORTECS C18+, 2.7 μm Column than on the competitor C18 column. The decreased peak width of the simulated API, imipramine, also reveals a smaller leading impurity peak that co-elutes with the main peak on the competitor solid-core column. The narrower peak shape of the Amitriptyline peak allows for better characterization of the simulated impurity, including determining peak area, and ultimately a quantitative result.
The reliable and accurate analysis of impurity peaks can also be achieved via mass spectrometry. During the above separations, the impurity peak (m/z) 278.4 amu, was examined. Figure 3 shows the SIR of the amitriptyline impurity on both the CORTECS C18+, 2.7 μm Column and the competitor solid-core C18 column.
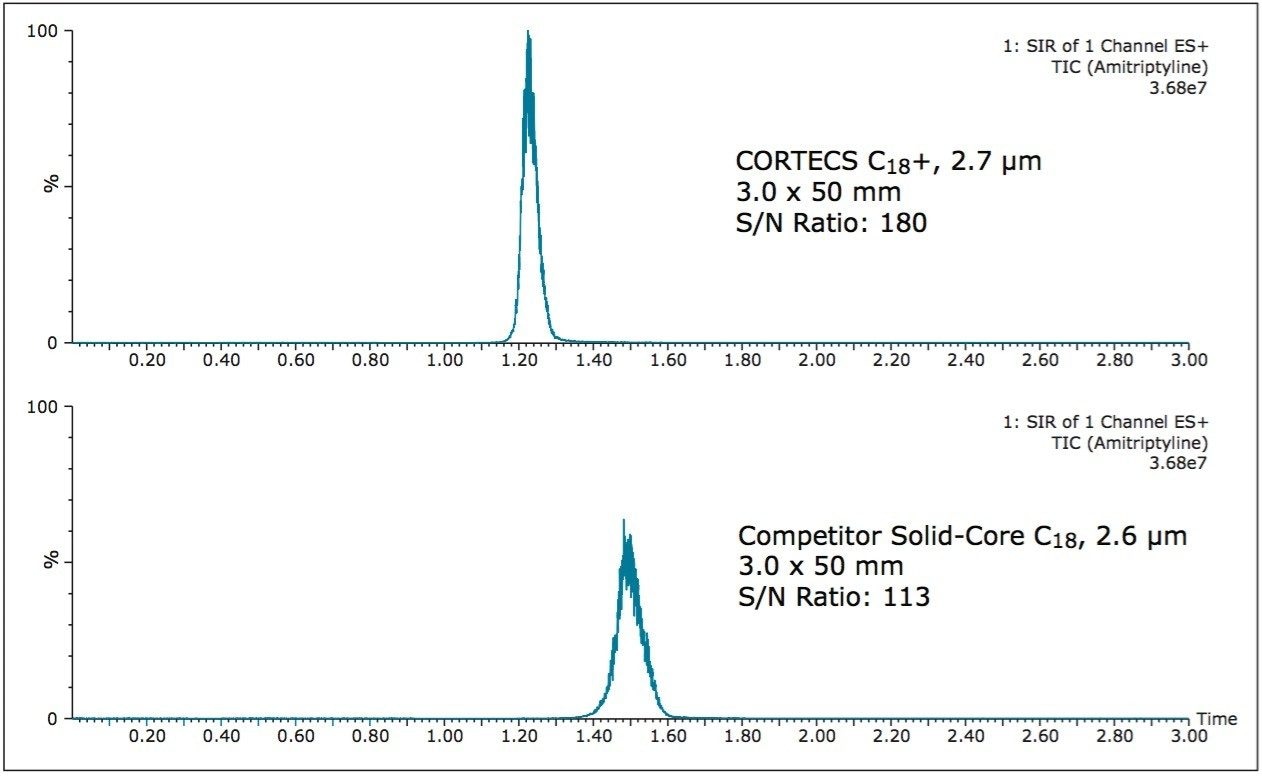
As Figure 3 shows, the impurity has a 60% higher S/N ratio on the CORTECS C18+, 2.7 μm Column compared to the competitor solid-core C18, 2.6 μm column. A higher S/N ratio gives the analyst a better signal leading to more accurate data analysis and detection of peaks at lower concentrations. Additionally a higher signal allows an analyst to get mass information on low level, unknown impurities leading to better characterization of the sample.
Low level impurity testing is important in a wide variety of industries including pharmaceutical manufacturing. Some validated USP monographs require that a formulated sample have less than a certain level of impurities. These levels vary based on the analysis being performed. In order for an analyst to accurately and reliably determine the level of impurities, the individual impurity peaks must be separated. CORTECS C18+, 2.7 μm Columns contain solid-core, charged surface particles which allow rapid separation of basic compounds while offering superior peak shape and MS signal when compared to competitor solid-core columns. The use of CORTECS C18+, 2.7 μm Columns for routine analysis of basic compounds allows analysts to use MS compatible mobile phases leading to better MS signals, which can increase the quality of the characterization of low level impurity samples. Additionally, CORTECS C18+, 2.7 μm Columns can lead to more reliable integration in UV analysis by reducing peak width and increasing resolution. The superior peak shape and enhanced signal allows better characterization of peaks with less method development effort, leading to higher analyst productivity.
720005062, May 2014