The goal of this study is to identify the presence of the cannabinoids cannabinol (CBN), cannabidiol (CBD), and Δ9 tetrahydrocannabinol (Δ9-THC) in Cannabis sativa using High Performance Thin Layer Chromatography (HPTLC) separation and confirmation with ACQUITY QDa Detector.
Cannabinol (CBN), cannabidiol (CBD), and Δ9-tetrahydrocannabinol (Δ9-THC) are the most studied active ingredients in Cannabis sativa and are used medicinally to treat several health disorders such as migraine, epilepsy, and appetite loss.
Cannabinol (CBN), cannabidiol (CBD), and Δ9-tetrahydrocannabinol (Δ9-THC) are the most studied active ingredients in Cannabis sativa, and are used medicinally to treat several health disorders such as migraine, epilepsy, and appetite loss. The cannabinoids can be present at varying concentrations in the plant depending on the specific strain and growing conditions. It is therefore essential to confirm their presence for quality control and to ensure appropriate administration to the patient.
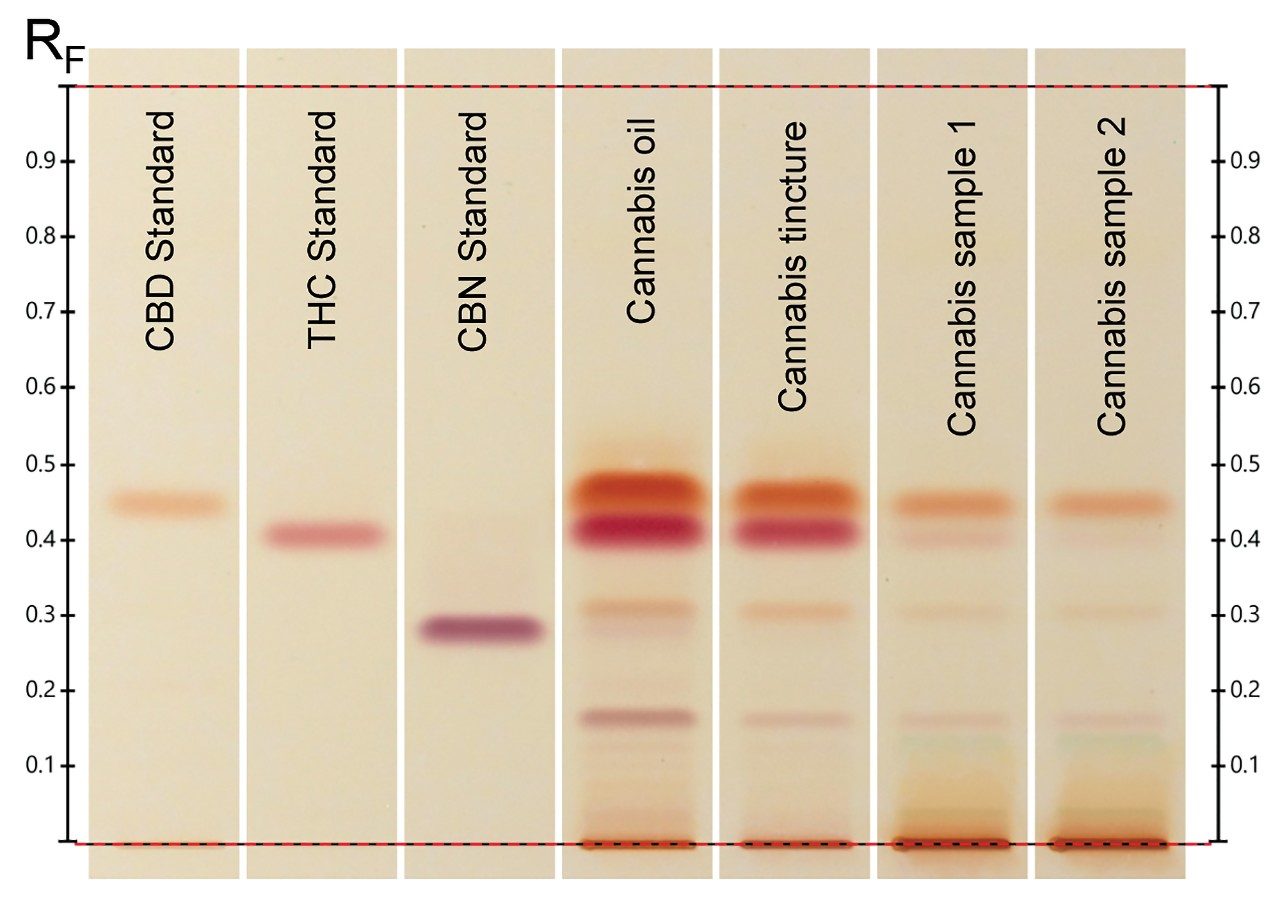
Thin layer chromatography (TLC) is an analytical separation technique where separation occurs on an open stationary phase layered on a support such as glass or plastic. HPTLC is the semi- or fully-automated sample application, plate development, and quantitative analysis technique. Absolute confirmation of contaminants separated by TLC is done using the ACQUITY QDa by directly interfacing the plate to the mass detector source inlet. The ACQUITY QDa is easy to use and offers higher selectivity for co-eluting compounds than UV alone and provides more confidence in the analysis. For compounds that have poor UV absorbance, derivitization is necessary for sample analysis. Mass detection can bypass the extra analysis steps and measure the sample directly. Standards were provided by Lipomed AG (Arlesheim, Switzerland) with CBN and CBD at a concentration of 1 mg/mL in methanol and Δ9-THC at a concentration of 1 mg/mL in ethanol. Each stock solution was diluted 1:10 with methanol. Four cannabis products were obtained for analysis and prepared as follows. To start, 500 mg of each loosely ground sample is mixed with 10 mL of methanol, sonicated for 10 minutes, and then centrifuged for 5 minutes (2750 RCF). The supernatant is collected and used as test solution. Cannabis oil and cannabis tincture are diluted 1:10 with methanol. Next, 5 µL of each standard, 2 µL of cannabis tincture, 5 µL of cannabis oil, and 8 µL of each Cannabis sample were spotted onto an HPTLC Si 60 F254, 20 x 10 cm plate by the Automatic TLC Sampler 4 and developed in Cyclohexane – di-isopropyl ether – diethyl amine 52:40:8 (according to, System B).¹ The plate was derivatized by immersion into Fast Blue salt B for five seconds using the Chromatogram Immersion Device and then allowed to dry before visualization under white light.
Target zones are directly eluted using the TLC-MS Interface 2 with oval elution head into the ACQUITY QDa at a flow rate of 0.5 mL/min with 0.1% ammonium hydroxide. The ACQUITY QDa is operated in negative electrospray ionization mode using the following settings: 0.8 kV capillary voltage, 15 V cone voltage, and 600 °C desolvation temperature. A full scan spectrum with the range of 50 - 650 m/z is acquired at a sampling rate of 10.0 points/sec (continuum). Data processing and evaluation of mass spectra are performed with Empower Chromatography Data Software. Figure 1 shows the ACQUITY QDa mass detection results from HPTLC separation (Figure 2) of CBN standard as well as CBD and THC from a Cannabis oil sample.
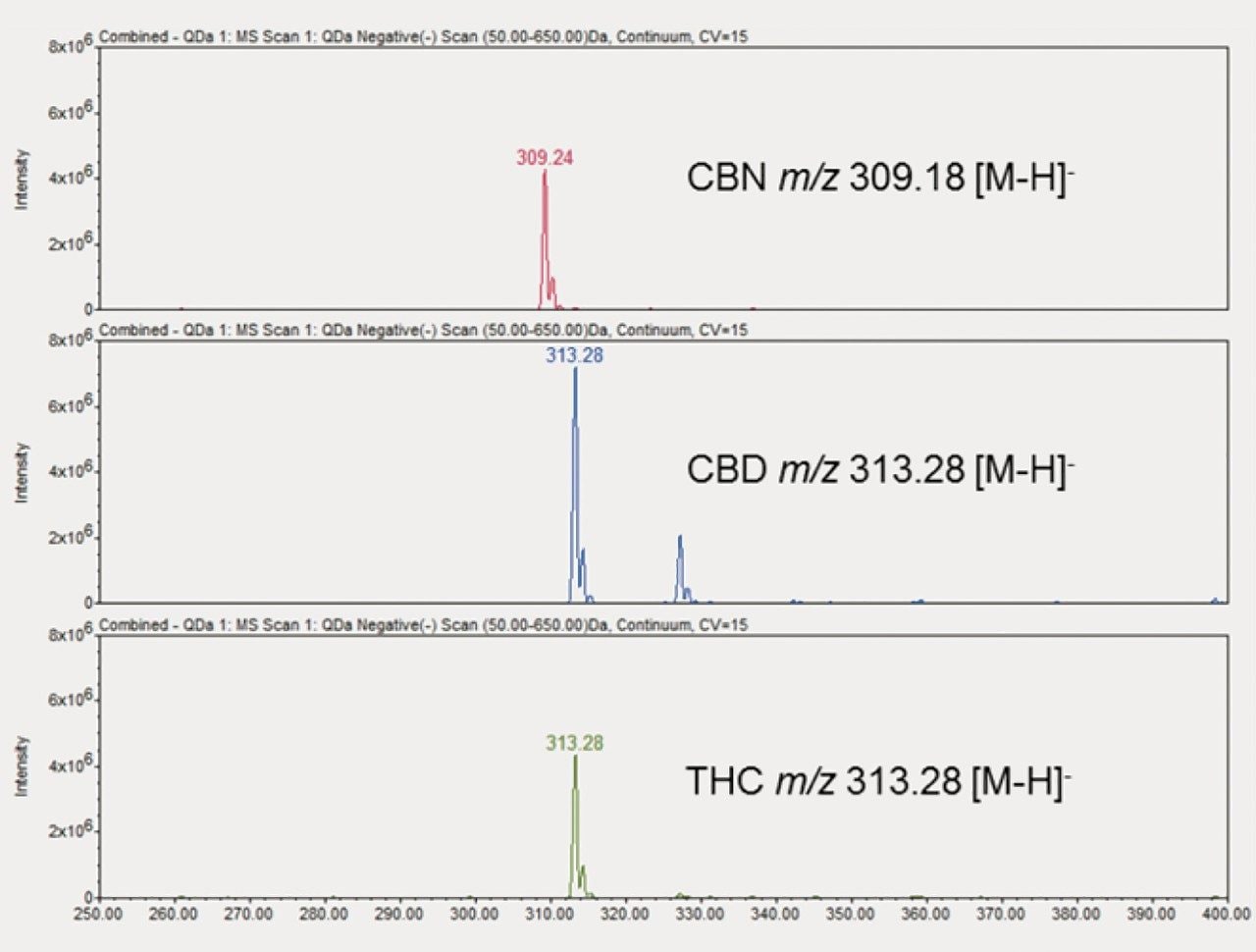
An HPTLC-MS method for the detection and confirmation of the cannabinoids CBD, CBN, and THC has been developed and applied to cannabis oil, cannabis tincture, and two Cannabis plant samples. The mass detection of the key cannabinoids using the ACQUITY QDa Detector increases the efficiency of developing better robust and reliable methods and complements traditional HPTLC-UV methods.
720006047, July 2017