For research use only. Not for use in diagnostic procedures.
This application highlights a simple sample pretreatment and SPE sample preparation strategy combined with analytical flow LC and tandem quadrupole MS for the highly sensitive and reproducible analysis of liraglutide from human plasma. Protein precipitation in combination with a mixed-mode SPE performed in the μElution format eliminated the need for sample evaporation, reducing losses due to adsorption, and provided selectivity to the assay.
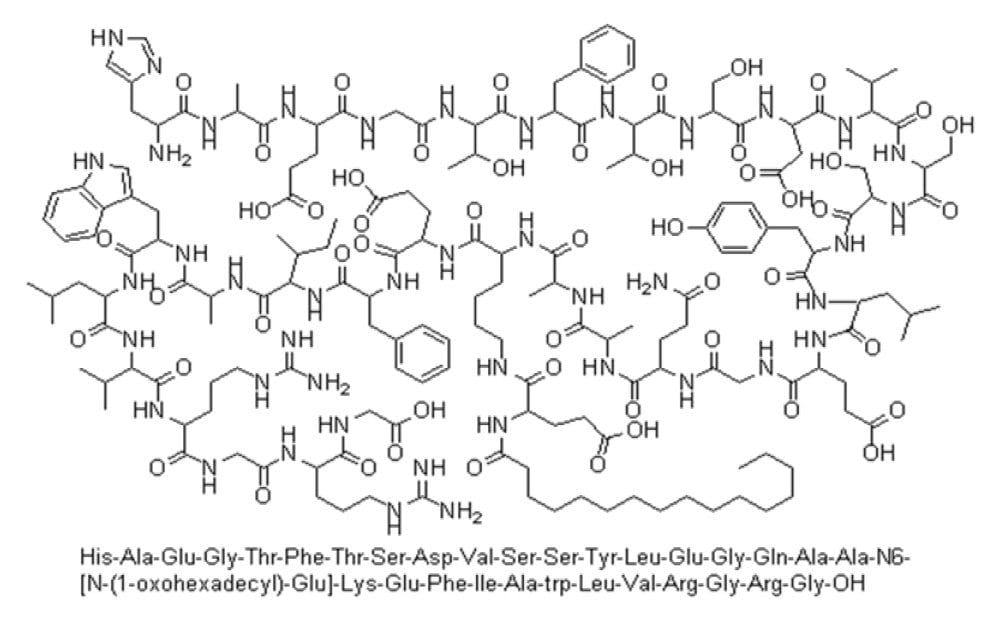
Native human glucagon-like peptide 1 (GLP-1) is a 30-amino acid peptide released from the gut in response to meals and has the capacity to regulate insulin secretion, exert extra pancreatic glucoregulatory actions and affect appetite and food intake.1-3 However, with its very short half-life (t1/2 = 1–2 min), its usefulness for the treatment of type 2 diabetes is limited. Hence GLP-1 receptor agonists with improved pharmacokinetic properties are now being developed as a new class of antidiabetes drugs. Liraglutide (ViCTOZA) is a therapeutic peptide, consisting of 31 amino acids with a molecular weight of 3751 (Figure 1). It is a human glucagon-like peptide 1 (GLP-1) analogue with high sequence homology to native GLP-1 and is used in the treatment of type 2 diabetes.4 It is administered once-daily as an isotonic solution by subcutaneous injection. With delayed absorption, plasma protein binding, and stability against metabolic degradation from endogenous enzymes, liraglutide’s pharmacokinetic profile is greatly better than endogenous GLP-1, Cmax (35 ng/mL), t1/2 >13 hrs, and Tmax of 9–13 hrs.5-7
With the first patent for Victoza expiring in August 20178 and subsequent patent expirations for this drug drawing closer, research and development of liraglutide, biosimilars, and next generation insulin therapies has increased. Thus, there is also an increased need for a sensitive and selective analytical method for its quantification. With fast method development times and high specificity, LC-MS/MS assays for peptide quantification have become increasingly popular. Compared to small molecule, method development and accurate LC-MS quantification of peptides, like liraglutide, can be challenging. MS sensitivity is often lower due to poor ionization, insufficient transfer into the gas phase, poor fragmentation, and obtaining selectivity is often complicated due to interferences from biological matrices. The work described here uses UPLC separation, tandem quadrupole MS, and selective sample preparation to achieve a lower limit of quantification (LLOQ) of 1 ng/mL extracted from plasma.
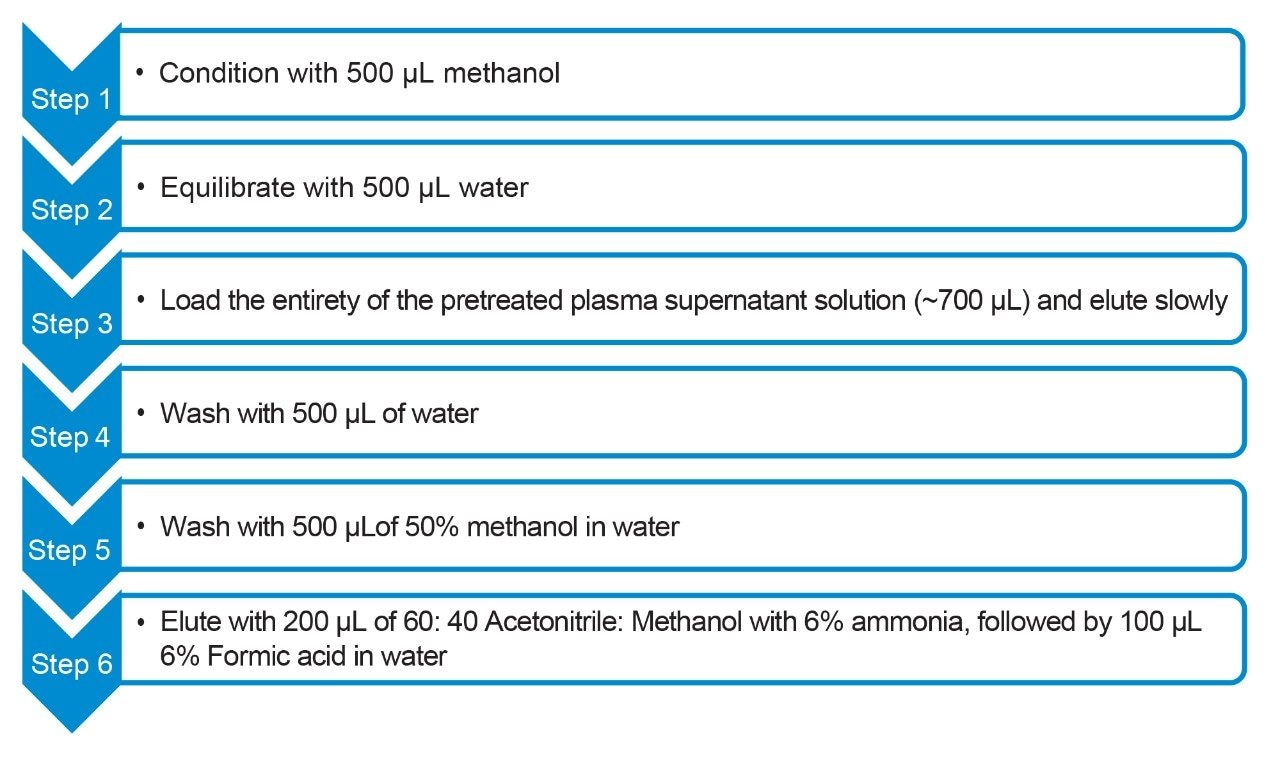
Standards and quality control (QC) samples were prepared by spiking liraglutide into commercially available human plasma at various concentration levels (0.5–200 ng/mL). Calibration curve standards were prepared in duplicate, while five replicates were prepared for each QC level. A 300 µL aliquot of each of the prepared plasma standards and QC samples was pretreated with 200 µL of acetonitrile and 200 µL of Milli-Q water, and vortexed for one minute, followed by centrifugation at 15 °C with 4500 rpm for 10 min. The resulting supernatant (700 µL) was then extracted using an Oasis WAX µElution Plate with the protocol shown in Figure 2.
|
LC system: |
ACQUITY UPLC I-Class System |
|
Column: |
ACQUITY UPLC Peptide BEH C18, 300 Å, 1.7 μm, 2.1 × 150 mm [p/n: 186003687] |
|
Column temp.: |
80 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
5.0 μL |
|
Mobile phase A: |
0.3% Formic acid in water |
|
Mobile phase B: |
Acetonitrile:methanol 50:50 |
|
Mass spectrometer: |
Xevo TQ-XS Tandem Quadrupole, ESI+ |
|
Capillary voltage: |
3.0 KV (+), |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
550 °C |
|
Cone gas flow: |
150 L/h |
|
Desolvation gas flow: |
1000L/h |
|
Collision cell pressure: |
3.8 × e-3 mbar |
|
Chromatography software: |
MassLynx |
|
Quantification software: |
TargetLynx |
In this work, we have developed a complete sample preparation and UPLC LC-MS/MS method for sensitive and accurate quantification of liraglutide from plasma. The method incorporates a mixed-mode SPE extraction using Oasis WAX in the 96-well µElution Plate format [p/n: 186002500]. Oasis WAX is a polymeric sorbent, with both reversed phase and weak anion exchange retention mechanisms, which provides orthogonality to the reversed phase LC separation for improved selectivity, while the µElution format facilitates high throughput extraction and sample concentration without the need for evaporation, reducing liraglutide losses due to adsorption and non-specific binding.
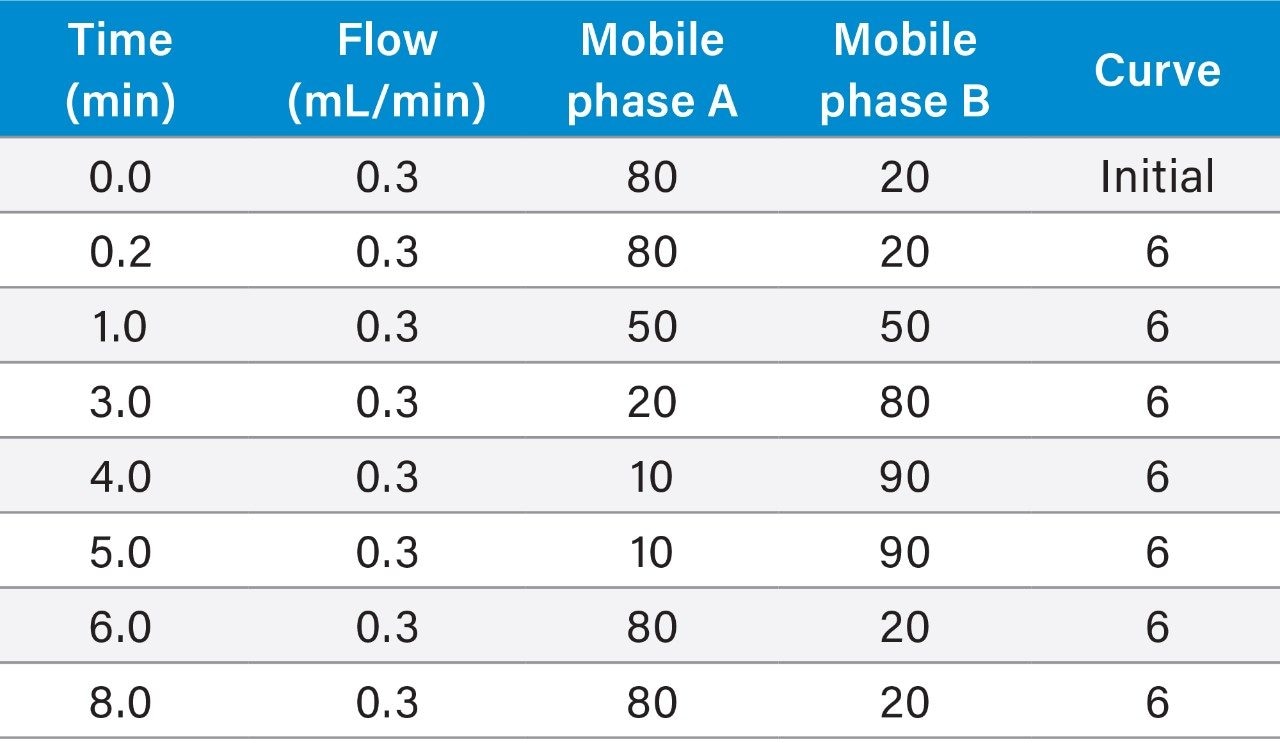
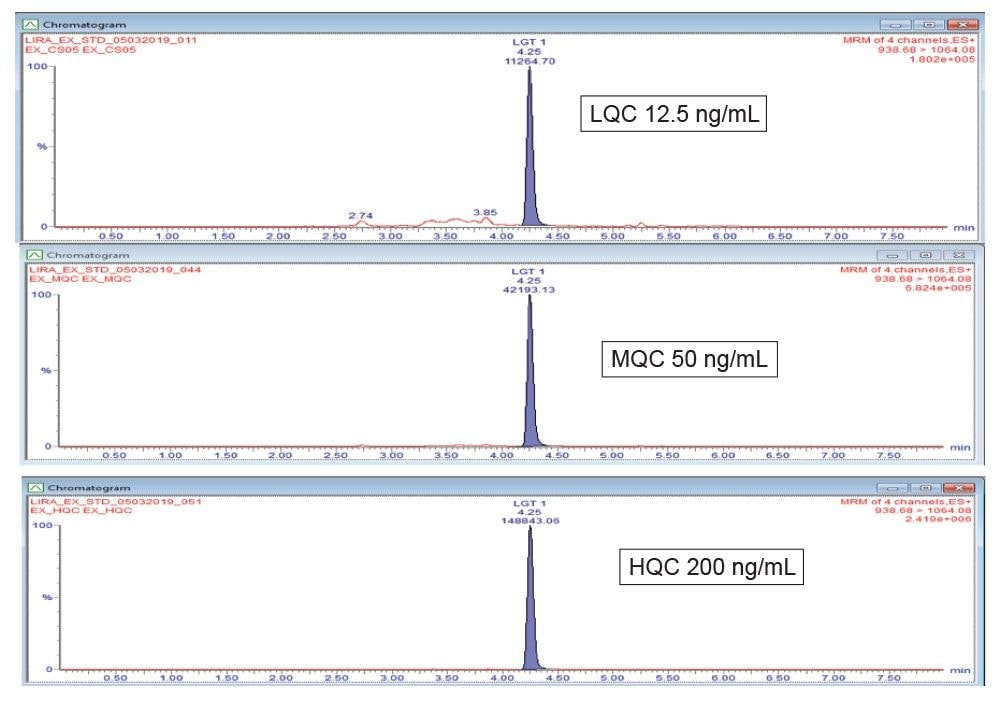
A simple, analytical scale LC method was developed using an ACQUITY UPLC I-Class System. Chromatographic separation was achieved with an ACQUITY UPLC Peptide BEH C18, 300 Å, 1.7 µm, 2.1 × 150 mm Column, using a linear gradient (Table 1) with 0.3% formic acid in water and acetonitrile: methanol (50:50) mobile phases at a flow rate of 0.3 mL/min. Total cycle time was 8 minutes. Use of the low dispersion ACQUITY UPLC I-Class System and sub-2-µm BEH C18, UPLC Column provided narrow peak widths (<8 seconds), affording a S/N of 126 for the lower limit of quantification (LLOQ) 0.5 ng/mL extracted plasma sample. The sensitivity and selectivity of this SPE-LC-MS/MS is illustrated in Figure 3. Specifically, for liraglutide, use of a larger angstrom pore size column in combination with sub-2 µm particles size, slower flow rate, and shallow gradient, facilitated better diffusion for improved peptide recovery, resolution from endogenous interferences, and reduced carryover.

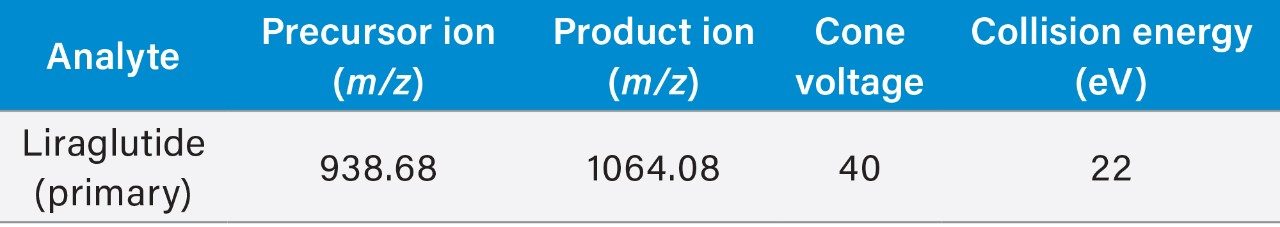
MS analysis was performed using the Xevo TQ-XS Tandem-Quadrupole MS (ESI+). The 4+multiply charged precursor for liraglutide at m/z 938.68 and selective fragments at m/z 1064.08 (Y ion) were chosen for MRM quantification. Although many peptides produce intense fragments below m/z 200, these ions (often immonium ions) result in high background in extracted samples due to their lack of specificity. In this assay, the use of highly specific y-ion fragments above m/z 1000 yielded significantly improved specificity, facilitating the use of simpler LC and SPE methodologies. Optimized MS conditions used for liraglutide quantification are summarized in Table 2.
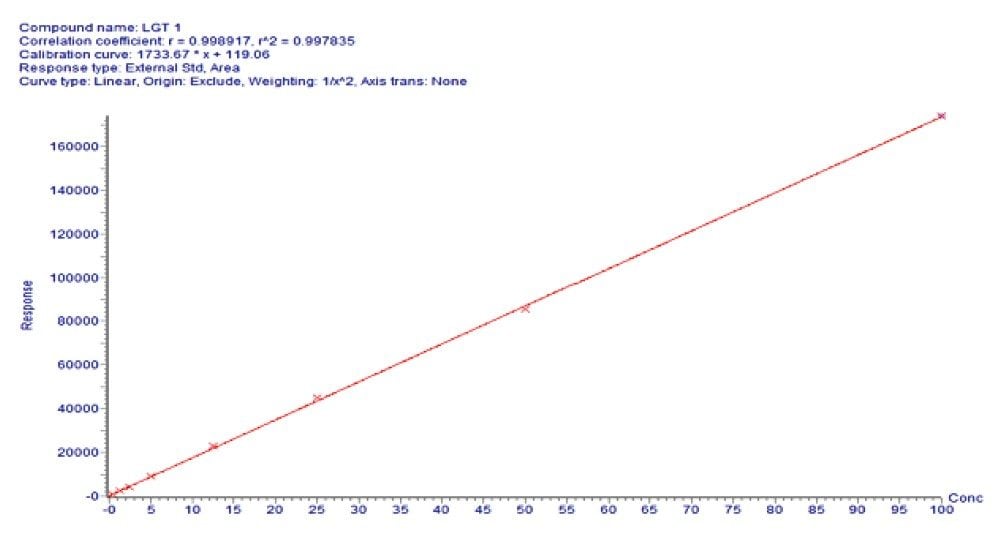
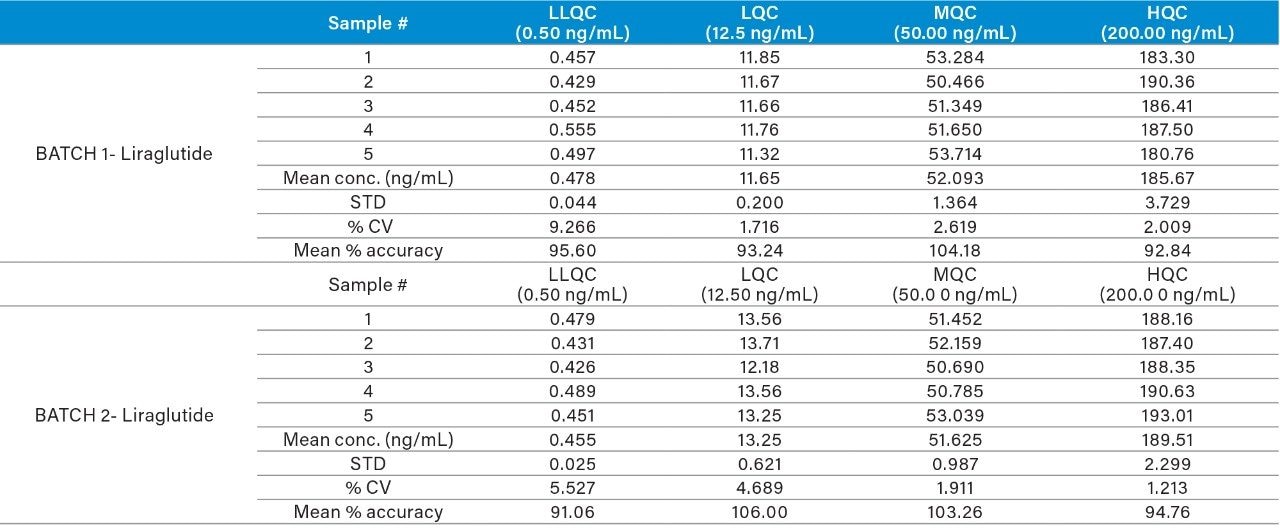
Using this complete SPE LC-MS method, quantitative performance was excellent. Calibration curves were linear (r2>0.995)1 from 0.5–200 ng/mL with accuracies between 85–115% and CVs ≤15% for all points on the curve. Figure 4 illustrates this performance. At the same time, QC statistics easily met recommended bioanalytical method development guidelines with average accuracy values between 93–106% and excellent precision (CVs ≤9%). This QC performance is highlighted in Table 3 for precision and accuracy (PA) batches, while chromatographic performance for the low, mid, and high QCs is illustrated in Figure 5.
This application highlights a simple sample pretreatment and SPE sample preparation strategy combined with analytical flow LC and tandem-quadrupole MS for the highly sensitive and reproducible analysis of liraglutide from human plasma. Protein precipitation in combination with a mixed-mode SPE performed in the µElution format eliminated the need for sample evaporation, reducing losses due to adsorption, and provided selectivity to the assay.
Use of a sub-2-µm particle C18 column with larger angstrom pore size in combination with an optimized LC method provided excellent chromatographic performance and resolution from endogenous interferences. The analytical sensitivity (0.5 ng/mL), linear dynamic range (0.5–200 ng/mL), and excellent reproducibility of the method described reliably measures liraglutide from only 300 µL of plasma. This developed method has demonstrated its fit-for-purpose use in support of drug discovery and research.
720006568, May 2019