Metrological Traceability of the Waters MassTrak Endocrine Steroid Calibrator and Quality Control Sets
For in vitro diagnostic use. Not available in all countries.
Abstract
LC-MS/MS methods in clinical laboratories are often based on validated laboratory developed tests (LDTs), which comply with local regulatory guidelines and international standards. In some geographies, laboratories are required to use metrologically traceable calibration materials to aid in compliance with ISO 15189:2012 Medical laboratories–Requirements for quality and competence. Therefore, metrological traceability has been incorporated in to the design, development, and manufacture of the Waters MassTrak Endocrine Steroid Calibrator and Quality Control Sets (IVD), aiding laboratories in their compliance to ISO 15189, and providing confidence in the accuracy and harmonization of results when using validated LC-MS methods.
In this application brief, we provide an overview of the Waters MassTrak Endocrine Steroid Calibrator and Quality Control Sets and their accuracy and comparisons across lot-to-lot testing.
Benefits
- Metrologically traceable calibrators and QCs that aid laboratories in their compliance to ISO 15189
- Confidence in the accuracy steroid hormones and provides a path to laboratory method harmonization
- Lyophilized calibrators and QCs that reduce sample preparation time
Introduction
LC-MS/MS methods in many clinical laboratories are based on Laboratory Developed Tests (LDTs), which can involve significant manual preparation in the pre-analytical workflow. This can include the preparation and characterization of in-house calibrator and QC samples by the laboratory technician, which may result in errors and inaccuracies of the materials, leading to reductions in lab efficiency. In addition, to help adhere to local regulatory guidelines and international standards such as ISO 15189, there is a need for metrologically traceable materials to improve the accuracy of results. The use of metrologically traceable materials for calibration of LC-MS/MS methods will also provide a pathway towards laboratory harmonization, particularly if these materials are manufactured to high standards with minimal variability across different manufacturing lots.
The Waters MassTrak Endocrine Steroid Calibrator and Quality Control Sets (IVD) (Figure 1) contains a range of steroid hormones in lyophilized serum that have been sourced to obtain the highest level of metrological traceability available. Cortisol, testosterone, 17-hydroxyprogesterone, and progesterone are value assigned by reference measurement procedures. Dehydroepiandrostenedione sulfate (DHEA-S), 21-deoxycortisol, corticosterone, 11-deoxycortisol, androstenedione, 11-deoxycorticosterone, dehydroepiandrosterone (DHEA), and dihydrotestosterone (DHT) are gravimetrically prepared from certified reference material in stripped serum. All steroid hormone concentrations were confirmed with independent QCs and proficiency testing (PT) or External Quality Assessment (EQA) schemes where available.
Results and Discussion
Improvements in Laboratory Efficiency
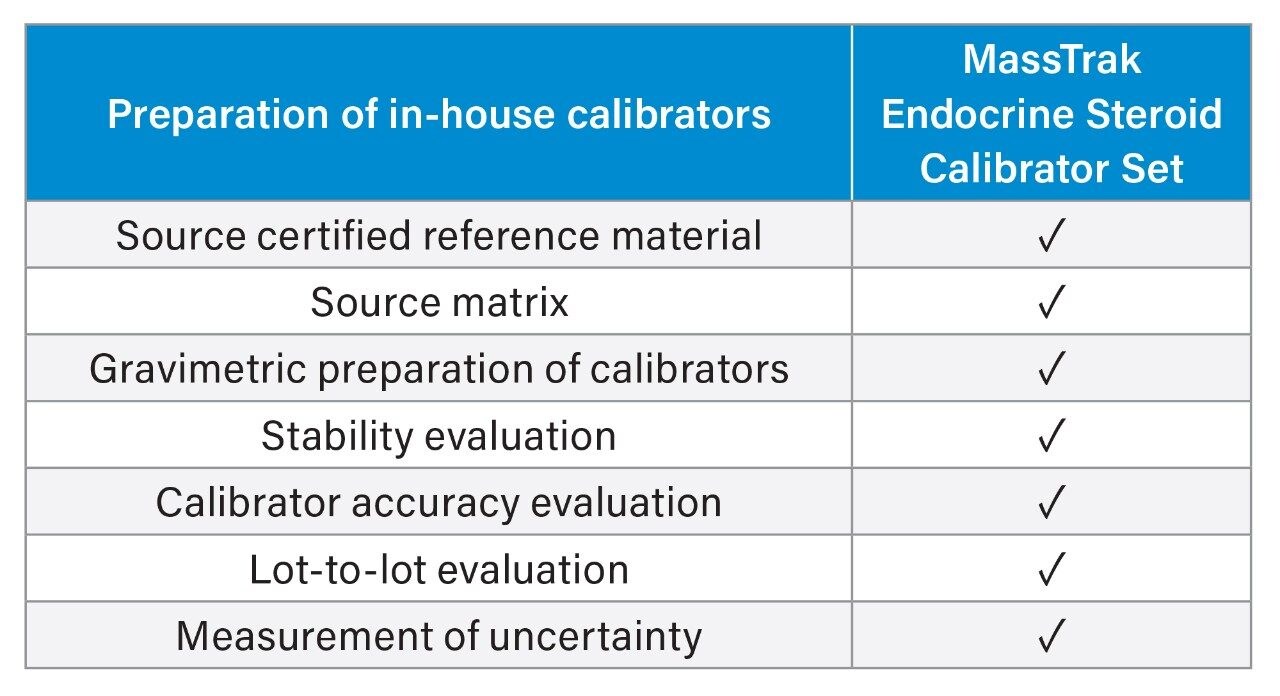
The MassTrak Endocrine Steroid Calibrator and Quality Control Sets have been designed and manufactured to assist laboratories in their compliance with ISO15189. A key benefit is the improvements in laboratory efficiency gained from using ready to use (following reconstitution) metrologically traceable calibrator and QC materials. Table 1 highlights the significant time and resource savings made when using commercial calibrators and QCs, by eliminating the multiple steps a laboratory performs when preparing in-house calibrators.
Metrological Traceability
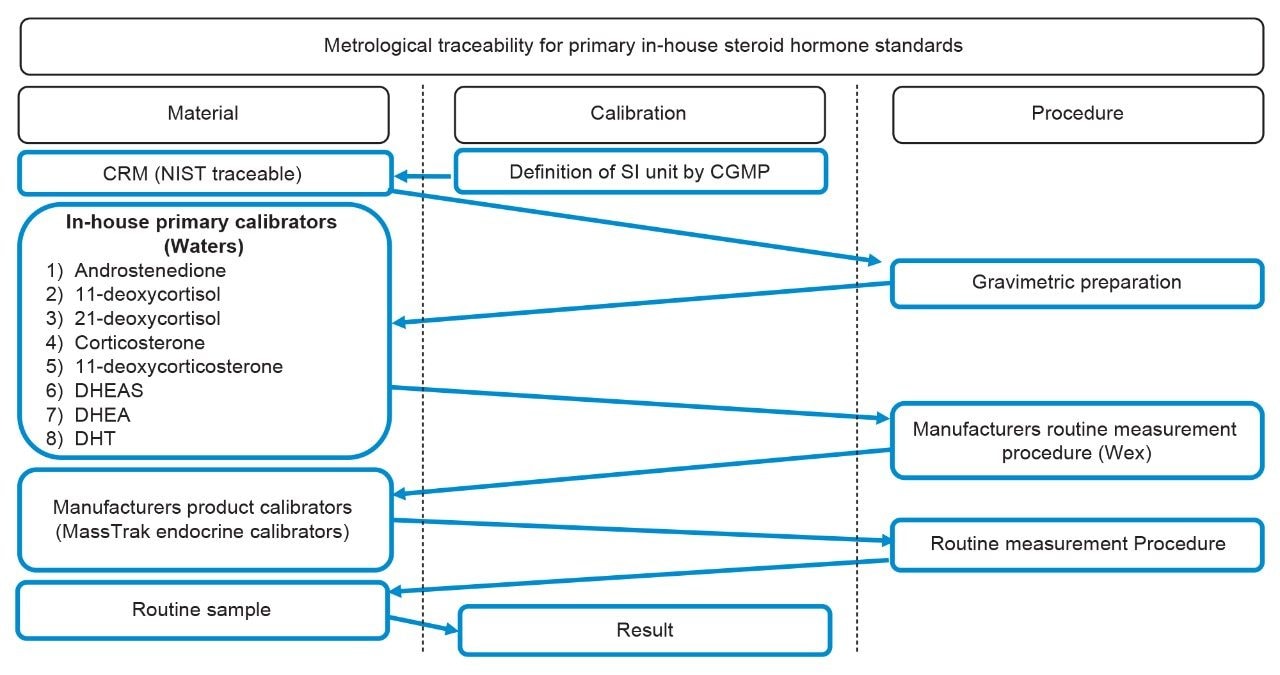
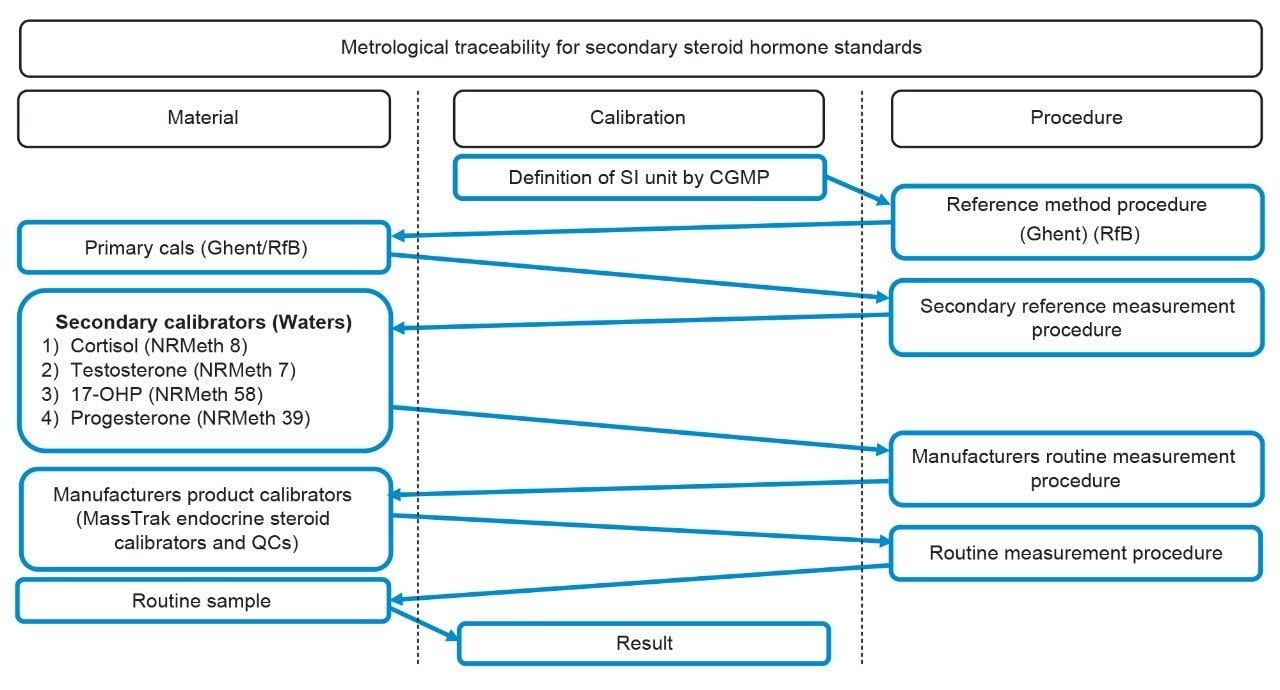
The metrological traceability associated with the MassTrak Endocrine Steroid Calibrator and Quality Control Sets uses two processes. Primary in-house standards for androstenedione, 11-deoxycortisol, 21-deoxycortisol, corticosterone, 11-deoxycorticosterone, DHEA-S, DHEA, and DHT are traceable to CRM (NIST traceable) material with values based on gravimetric assignment (Figure 2). Secondary in-house standards for cortisol, testosterone, 17-OHP, and progesterone are traceable to the University of Ghent and Rfb reference measurement procedures with values based on a secondary reference measurement procedure assignment (Figure 3). The primary and secondary standards are used to generate and assign concentrations to the MassTrak Endocrine Steroid Calibrator and Quality Control Sets.
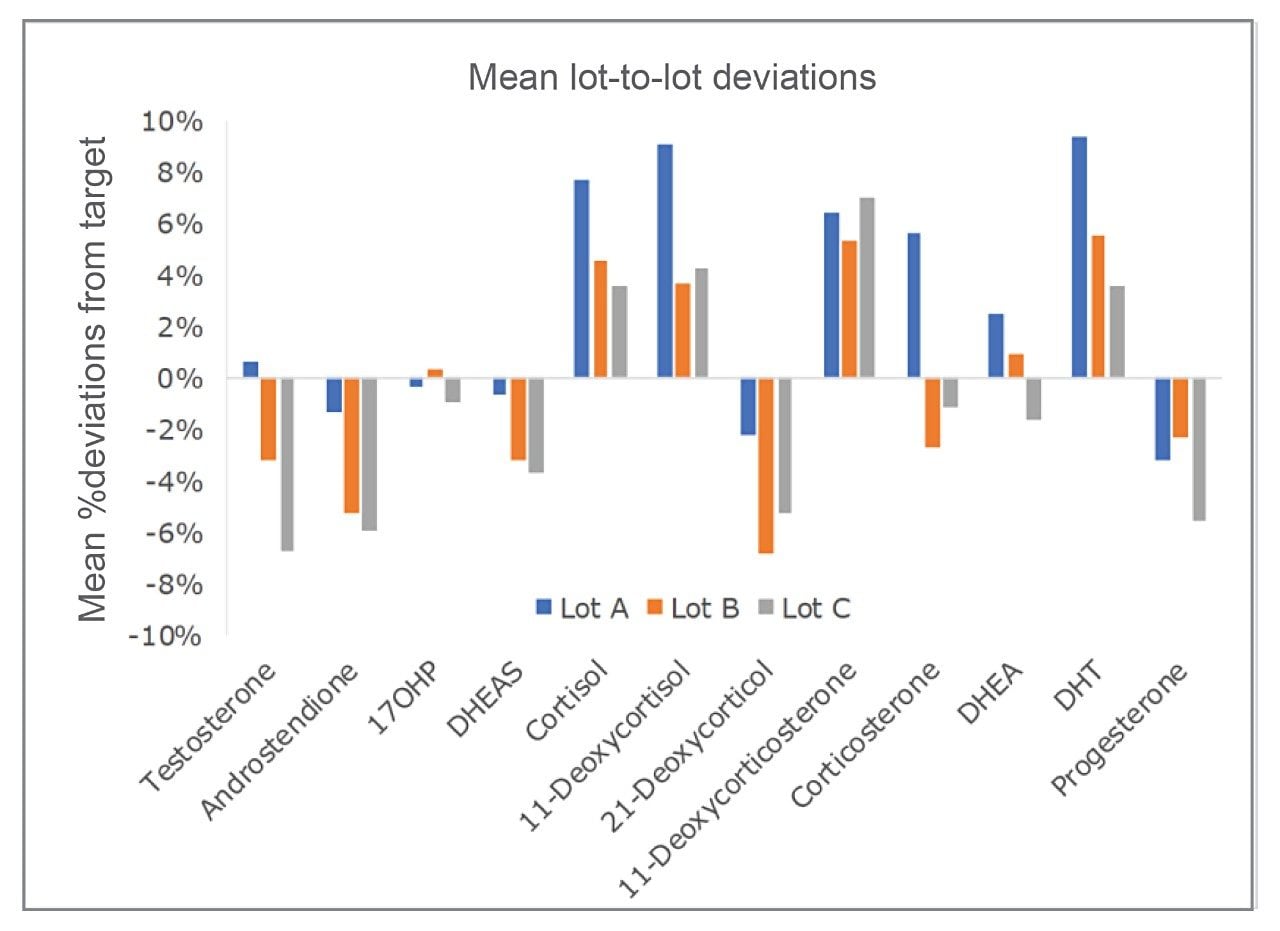
Accuracy and Lot-to-Lot Comparisons
The accuracy of the calibrator set was determined through evaluation of in-house QC and External Quality Assessment (EQA) samples. Three lots of manufacturing material for the calibrators were evaluated for accuracy and mean deviations from the assigned values at low and high concentrations following LC-MS/MS analysis using the routine measurement procedure. Results are shown in Figure 4. This data not only demonstrates the accuracy of the calibrators but also the lot-to-lot reproducibility of the manufacturing process, which is important if laboratories seek to maintain their harmonization standards overtime using different manufacturing lots.
Conclusion
Metrological traceability of the MassTrak Endocrine Steroid Calibrator and Quality Control Sets has been established aiding laboratories in their compliance to ISO 15189. The assay’s accuracy and precision have been confirmed through the use of independent QCs and proficiency testing (PT) schemes.
Disclaimer
MassTrak Endocrine Steroid Calibrator and Quality Control Sets are not available for sale in all countries. For information on availability, please contact your local sales representative.
720007404, Revised April 2022