Reliable High Resolution Protein SEC Separations for Online Native LC-MS mAb Analysis
Abstract
Native liquid chromatography-mass spectrometry (LC-MS) is a powerful approach for protein analysis and size-exclusion chromatography (SEC) is one of the preferred analytical methods for such determinations. SEC should be an entropically driven separation wherein no interaction occurs between the analyte and the surfaces on the particle or on the packed column. In practice, this is difficult to achieve, particularly with the ammonium acetate mobile phases used in MS work.
Here we have explored SEC-MS with the use of ACQUITY Premier Protein SEC 250 Å columns that exhibit reduced undesired analyte-to-surface interactions. This new SEC column technology is based on Waters BEH particles containing a high coverage of hydroxy terminated polyethylene oxide (PEO) ligands contained within specialized column hardware featuring hydrophilic MaxPeak High Performance Surfaces. With this work, it is shown that these optimized surfaces confer several sought-after analytical capabilities, including improved limits of detection, more symmetrical peak shapes, and higher analyte recoveries. In the context of Native SEC-MS analysis of mAbs, these improved characteristics produce superior MS response and improved detection limits for the overall analysis.
Benefits
- Reduced undesired secondary interactions (ionic and hydrophobic) using MaxPeak High Performance Surfaces along with ACQUITY Premier Protein SEC 250 Å, 1.7 µm and XBridge Premier Protein SEC 250 Å, 2.5 µm hybrid organic-silica particles
- Use of lower concentration ammonium acetate mobile phases for optimal online MS detection
Introduction
Over the last decade, there has been increasing interest in performing native SEC separations with online MS detection. This combination is now used to routinely characterize size variants including both higher molecular weight aggregates and lower molecular weight fragments. For most methods, this entails the use of MS-compatible, ammonium acetate is a primary mobile phase component.1 MS-compatible ion exchange methods have also been effectively used to characterize intact and IdeS-digested monoclonal antibodies and to glean new information about mAb charge variants.2 A few researchers have even taken to optimizing MS-compatible conditions for hydrophobic interaction chromatography where surface accessible modifications provide sufficient retention differences to elucidate product variants in antibody drug conjugates.3
It is size exclusion chromatography (SEC) where one could argue there is the best fit for performing ammonium acetate-based separations and achieving higher throughput native LC-MS. With SEC, a sample can be rapidly desalted online, and in a few minutes between injections, it is possible to obtain high quality mass spectra. For native protein samples that retain in compact globular structures, an ammonium acetate LC-MS experiment produces simpler, lower charge state ions that are less prone to matrix interferences and in turn higher quality deconvoluted mass spectra.
While simple in concept, SEC can be a challenge to optimize for desired degrees of resolution, especially when restricted to the use of volatile mobile phase compositions.4 Goyon and co-workers explored SEC-MS considerations in 2017 and noted there to be chromatographic challenges with the use of ammonium acetate conditions.5 Distorted peaks and poor recovery were encountered even with the application of 100 mM ammonium acetate mobile phases. It was then proposed that SEC column technologies must improve to better facilitate this type of analytical work.
To that end, we have explored the use of a new SEC particle and column hardware technologies that exhibit fewer undesired secondary interactions. We have also applied this combined SEC column technology to the use of ammonium acetate mobile phase for native protein characterization. This new column technology is based on high coverage hydroxy-terminated polyethylene oxide (PEO) bonded particles and hardware built with hydrophilic MaxPeak High Performance Surfaces (Figure 1). With these unique properties, it is shown that the corresponding ACQUITY Premier and XBridge Premier Protein SEC 250 Å Columns can provide improved limits of detection, excellent peak shapes, and high recoveries when operated with MS-compatible ammonium acetate mobile phases.
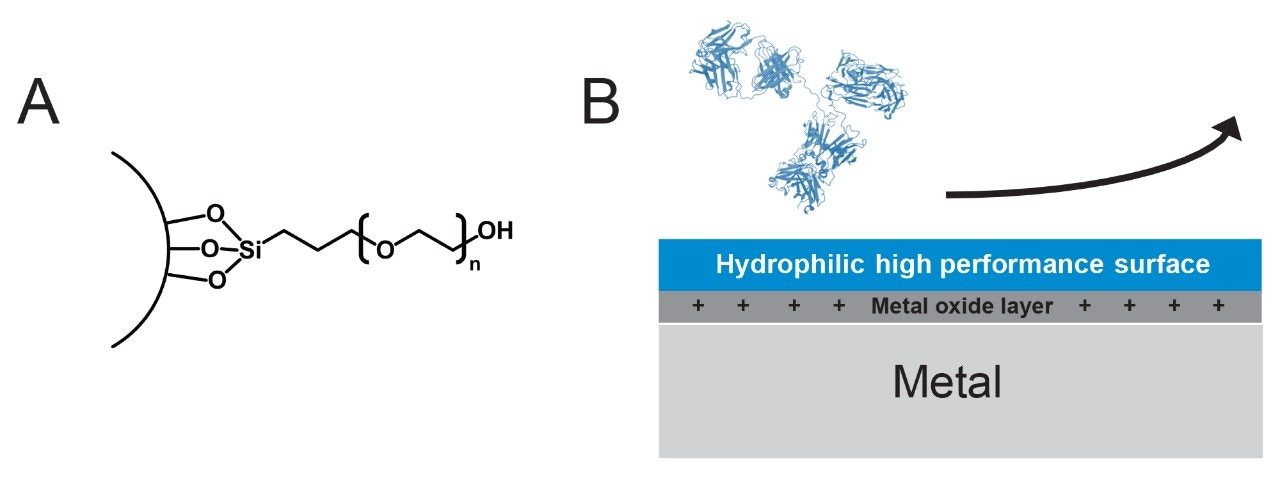
Experimental
Sample Information
NISTmAb reference material 8671 and Intact mAb Mass Check Standard (Waters p/n: 186006552) were diluted with water prior to SEC analysis according to the experimental details outlined below. The BEH200 SEC Protein Standard (Waters p/n: 186006518) was dissolved in 500 µL water. Intact mAb Mass Check standard was diluted in water. Mobile phases were prepared using Waters IonHance CX-MS pH Concentrates A and B (Waters p/n: 186009280 and 186009281). To perform a high sensitivity MS experiment, it is important to start with high quality mobile phases. Waters IonHance Concentrates are manufactured and quality control tested for this exact purpose. In this work, pH concentrates designed for cation exchange (CX)-MS separations are used to ratiometrically prepare an optimized pH and ionic strength for SEC-MS.
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class |
|
Detector: |
ACQUITY UPLC TUV Detector (with Titanium Flow Cell) |
|
Wavelength: |
280 nm |
|
Column: |
XBridge Premier Protein SEC 250 Å 2.5 µm, 4.6 x 150 mm Column (p/n: 186009959) ACQUITY Premier Protein SEC 250 Å 1.7 µm, 4.6 x 150 mm Column (p/n: 186009963) Commercially Available MeO-PEO Bonded SEC 200 Å 2.7 µm, 4.6 x 150 mm Column ACQUITY UPLC BEH SEC 200 Å 1.7 µm, 4.6 x 150 mm Column (p/n: 186005225) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
4 °C |
|
Injection: |
7.5 µL |
|
Flow rate: |
0.2 mL/minute |
|
Mobile phase A: (Figures 1, 3, and 4) |
1x IonHance CX-MS pH Concentrate A (50 mM Ammonium acetate, 2% ACN, pH 5) |
|
Mobile phase B: (Figures 1, 3, and 4) |
1x IonHance CX-MS pH Concentrate B (160 mM Ammonium acetate, 2% ACN, pH 8.5) |
|
Mobile phase mixing: (Figures 1, 3, and 4) |
40:60 A/B (116 mM Ammonium acetate, 2% ACN) |
|
Mobile phase A: (Figure 2) |
200 mM Ammonium acetate (pH 7; not titrated) |
|
Mobile phase B: (Figure 2) |
18.2 MΩ Water |
|
Mobile phase mixing: (Figure 2) |
Varied (see Figure 2 for details) |
MS Conditions
|
MS system: (Figures 1, 2, and 4): |
BioAccord/ACQUITY RDa Detector |
|
Mode: |
Full scan |
|
Polarity: |
Positive |
|
Cone voltage: |
150 V |
|
Mass range: |
High (400–5000 m/z) |
|
Scan rate: |
2 Hz |
|
Capillary voltage: |
1.5 kV |
|
MS system: (Figure 3) |
Xevo G2-XS QTof |
|
Acquisition window: |
As shown in Figure 3 |
|
Capillary voltage: |
2.5 kV |
|
Cone voltage: |
60 V |
Column Dimension Considerations
To optimize both LC and MS, 4.6 mm (versus 2.1 mm) I.D. columns have been used. SEC columns with a 4.6 mm I.D. can be optimally operated with a reasonably low flow rate of 0.2 mL/minute. This is a flow rate that can be accommodated by ionization sources, and it is a flow rate that will help ensure that LC system dispersion does not have too significant an effect on peak shape.
Results and Discussion
The assessment of ACQUITY and XBridge Premier Protein SEC 250 Å, 1.7 and 2.5 µm Column technology started with experiments to explore limits of detection. A mobile phase composition of 116 mM ammonium acetate was prepared through the online mixing of IonHance CX-MS pH concentrates A and B in a 40:60 (v/v) ratio. UV signal was then monitored for 7.5 µL injections of NISTmAb solutions that had been prepared through a serial dilution. Separations produced with a 0.2 mL/minute flow rate and a 4.6 x 150 mm XBridge Premier Protein BEH SEC 250 Å, 2.5 µm Column are shown in Figure 2A. With this column, NISTmAb could be detected by UV down to an injected quantity as low as 37.5 ng. These same experiments were performed with an alternative, commercially available column, namely a 4.6 x 150 mm column constructed from methoxy-terminated PEO bonded 200 Å, 2.7 µm particles and metallic hardware. Figure 2B displays the UV chromatograms obtained for the same series of mass load injections. While a discernable peak could be observed at 0.75 and 0.375 µg mass loads, it should be noted that they exhibited an appreciable amount of tailing. A poorly recovered peak was observed at a mass load of 0.15 µg, and no peaks could be discerned at mass loads of 75 and 37.5 ng. That an XBridge Premier Protein SEC 250 Å, 2.5 µm Column can produce well shaped peaks for NISTmAb down to the nanogram mass range when an ammonium acetate mobile phase is employed bodes well for its applicability to routine SEC/LC-MS particularly in applications such as clone screening, where sample limitation is commonplace.
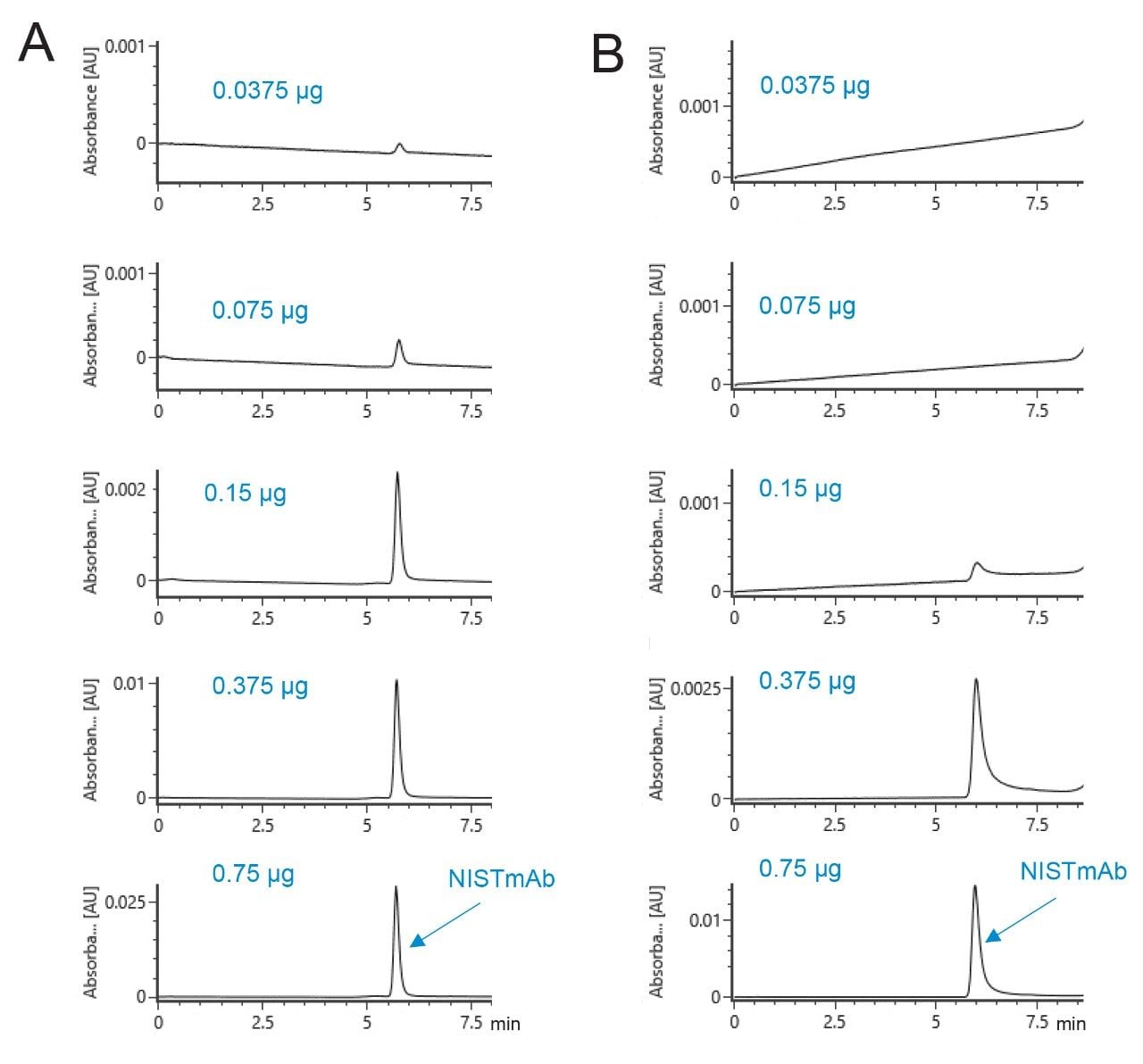
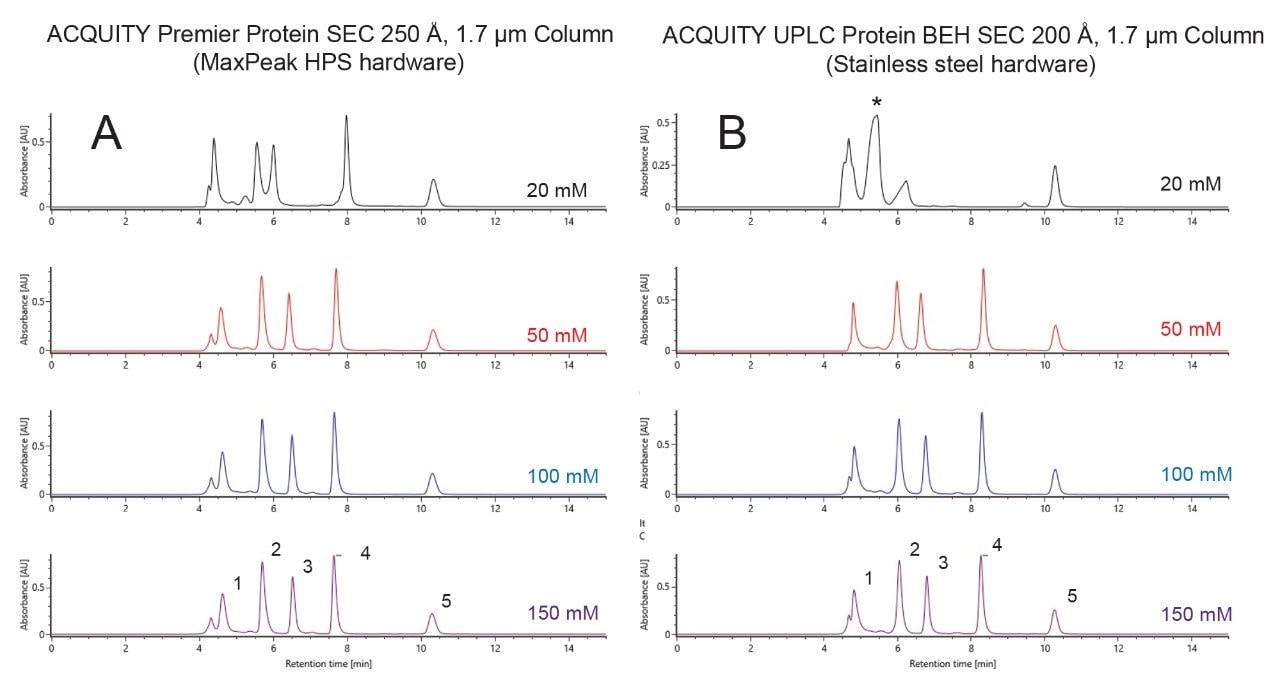
The dependence of ACQUITY and XBridge Premier Protein SEC 250 Å columns on the ionic strength of ammonium acetate mobile phases was investigated to assess the extent of residual ionic interactions. For this, a standard comprised of four different proteins, ranging in molecular weight from 10 up to 300 kDa, was analyzed as a function of mobile phase salt concentration. A 4.6 x 150 mm ACQUITY Premier Protein SEC 250 Å, 1.7 µm Column produced desired levels of peak resolution, peak shape, and recoveries with 50 mM and higher concentrations of ammonium acetate (Figure 3A). A mobile phase of only 20 mM showed slightly distorted peaks and higher peak-to-peak valleys. This suggests that a minimum requirement for this sample might exist somewhere between a 20 and 50 mM concentration. For comparison, these separations were repeated on a comparable particle size ACQUITY UPLC BEH SEC 200 Å, 1.7 µm Column, which is based on a diol bonded BEH particle and constructed using stainless steel hardware. The resulting separations showed a few noteworthy differences. The most significant of which was severe peak distortion seen with a 20 mM ammonium acetate separation. In this chromatogram, peaks were asymmetrical and there was a problematic co-elution of IgG and BSA. Use of a 50 mM mobile phase led to a significant improvement in column capabilities, but it remained a challenge to resolve the thyroglobulin dimer from the thyroglobulin monomer peak. Ultimately, it is the ACQUITY Premier Protein SEC 250 Å, 1.7 µm Column that proves to be better suited to the use of lower ionic strength mobile phases, which can be exploited to achieve higher sensitivity native LC-MS analyses.
Native SEC separations are now being routinely coupled to online electrospray ionization (ESI)-MS detection as a means to facilitate the deeper characterization of protein therapeutics, their non-covalent protein complexes, and size variants such as fragments. Our next study investigated the direct coupling of XBridge Premier Protein SEC 250 Å Columns with two different time of flight mass spectrometers, a QTof mass spectrometer (Xevo G2-XS QTof) and a compact TOF mass detector (BioAccord System comprised of an ACQUITY UPLC I-Class PLUS and an ACQUITY RDa Mass Detector).
All LC columns exhibit some level of background ions when coupled with mass spectrometry. ACQUITY and XBridge Premier Protein SEC columns have high coverage PEO bondings. As shown above, this affords higher recoveries and compatibility with lower concentrations of ammonium acetate in the volatile SEC mobile phase. It was noted that background ions between 600 and 900 m/z were observable, and these originate from the particle bonding. When performing native mAb SEC-MS, setting an acquisition window from 1000 m/z or higher will maximize TIC signal-to-noise by excluding this region. These interfering ion signals could potentially interfere with denaturing SEC analysis of mAbs or mAb fragments. For a denaturing SEC-MS experiment, it is recommended to use an ACQUITY UPLC Protein BEH SEC 200 Å Columns, as has been described elsewhere.6
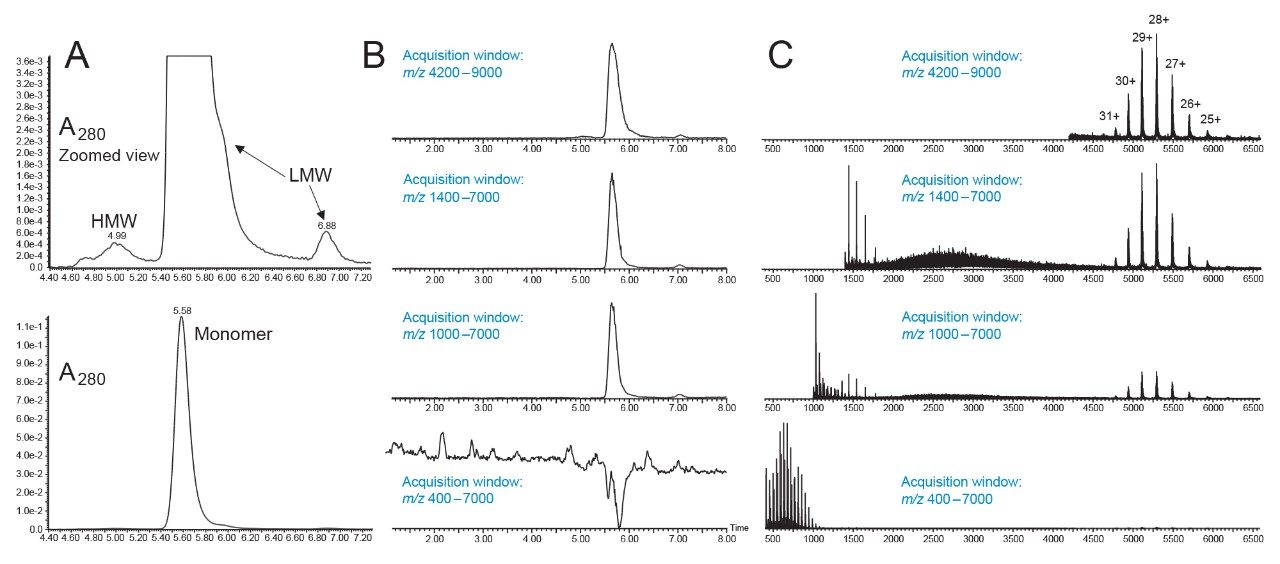
Figure 4 presents a collection of chromatograms and mass spectra that exemplify the quality of native mAb data that can be obtained with an XBridge Premier Protein SEC 250 Å, 2.5 µm, 4.6 x 150mm Column. A separation was performed with an approximately ~100 mM ammonium acetate mobile phase and the obtained UV chromatogram is shown in Figure 4A, where it can be confirmed that reasonable peak areas and recoveries for major size variant peaks were observed. The recovery of aggregates and high molecular weight species (HMWS) has been elusive for many previous implementations of online mass detection. Our results confirmed that the XBridge Premier Protein SEC 250 Å, 2.5 µm, 4.6 x 150 Column affords detection of distinct HMW peaks that are comparable in quality to an optimized non-volatile SEC mobile phase system. The HMW peaks in the Figure 4A chromatogram represent approximately 2% relative abundance, consistent with specifications of NISTmAb reference material. Next, Figure 4B shows the total ion chromatograms collected after UV detection using serial detection with a QTof mass spectrometer. In this case, the QTof was operated with various acquisition windows. On the bottom panel, a wide mass range from 400 to 7000 m/z was employed. The summed mass spectrum corresponding to the NISTmAb elution time is shown at the bottom of Figure 4C. The base peaks of the spectrum can be attributed to Δ44 Da PEO background ions. This is an expected result for the use of an ACQUITY or XBridge Premier Protein SEC 250 Å Column. With these ions, NISTmAb signal could not be readily observed through the analysis of the total ion chromatogram. Conversely, when the low mass limit of the acquisition window was set to 1000, 1400, and 4200 m/z, an increasingly more sensitive detection of protein species was achieved. Moreover, with an increase of the upper mass limit to 9000 m/z, some ion counts for the HMWS could be observed. For this experimental setup, an increase in mass load would be needed to obtain molecular weight on the HMW species.
SEC-MS analyses were also performed with a compact Tof instrument, namely the BioAccord System featuring an ACQUITY RDa Mass Detector. The ACQUITY RDa operates with one of two default mass range settings, including the high mass range acquisition setting (400 to 7000 m/z) that is the most appropriate selection for SEC-MS. Selected extracted ion chromatogram (XIC) mass ranges similar to the mass acquisition windows shown above can produce chromatograms that highlight the native mAb signal.
An example of a total ion chromatogram, obtained with a BioAccord System, is displayed in Figure 5A. Figure 5B presents the combined MS spectrum corresponding to the NISTmAb elution window. The zoomed XIC view of the 3000 to 7000 m/z range shows the appreciable signal for native NISTmAb.
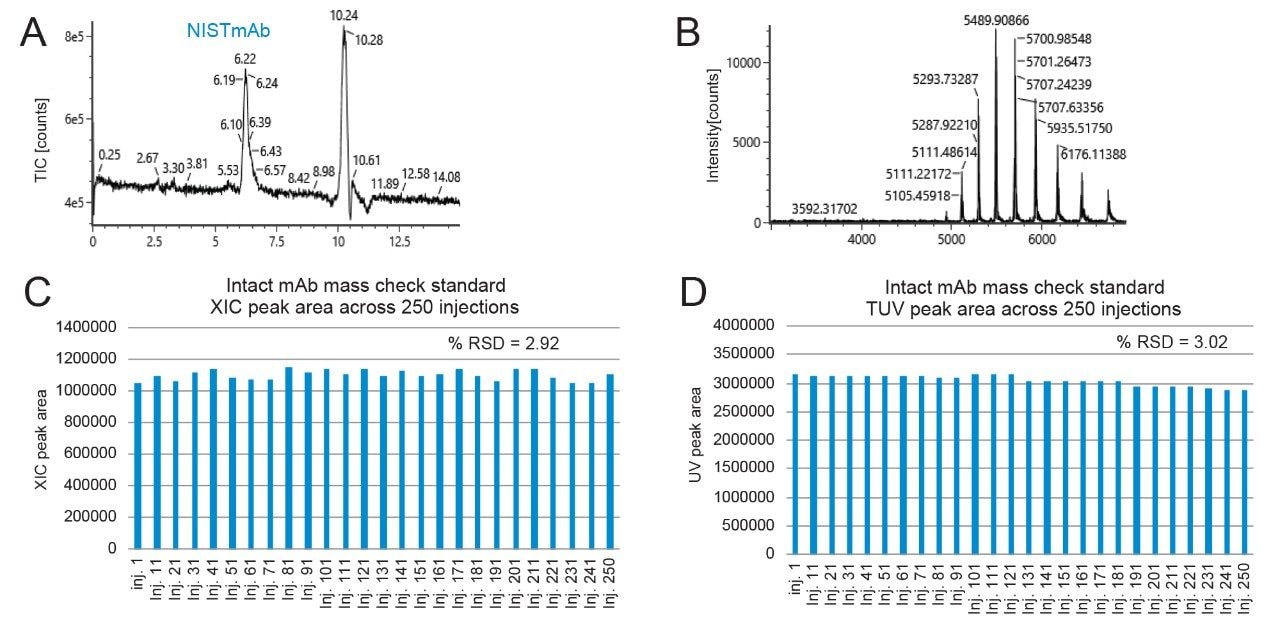
We investigated the stability of the BioAccord System with repeated injections of murine IgG (Intact mAb Mass Check Standard) and the XBridge Premier Protein SEC 250 Å, 2.5 µm Column. Here, 250 injections were made on the column, and SEC-MS runs were repeated for the entirety of the study without any intervening blank injections. Extracted mAb ion peak areas for every tenth run are summarized in Figure 5C. Protein MS signal was consistent from the start of the experiment through to the 250th injection. An RSD of only 2.9% was observed for the average across each of the reported injections. The peak areas observed by UV similarly showed minimal changes. These data thereby suggest that Premier Protein SEC Columns are fit-for-purpose for SEC-MS with ammonium acetate mobile phases.
Conclusion
Native LC-MS is a powerful approach for the analysis of protein therapeutics. With native LC-MS, lower charge states and, simpler charge state distributions, and lower matrix signal are obtained such that molecular weight information can be more easily deconvoluted from the acquired mass spectra. Native LC-MS can provide key information on size variants and it lends itself to the analysis of non-covalent interactions. Several different types of chromatography can be used for these types of analyses, but it is size exclusion chromatography (SEC) that offers one of the most conceptually and operationally simple forms of separation. SEC should ideally be an entropically driven separation wherein no interaction or adsorption occurs between the analyte and the surfaces within the column. In practice, these undesired interactions have been a challenge to minimize, particularly with the ammonium acetate mobile phases preferred for native LC-MS analysis. Many commercially available column technologies produce distorted peaks and poor recovery with the application of 100 mM ammonium acetate mobile phases, which has held analysts back from developing robust, higher sensitivity SEC-MS approaches.
Here, we have explored the use of a new SEC column technology that exhibits reduced levels of undesired secondary ionic and hydrophobic interactions under these MS-friendly separation conditions. This new column technology is based on a high coverage hydroxy-terminated polyethylene oxide (PEO) bonded particle and complemented by column hardware built with hydrophilic MaxPeak High Performance Surfaces to minimize analyte interactions with metallic column hardware. In this work, these unique surfaces frequently sought-after SEC-MS capabilities, including improved limits of detection, more symmetrical peak shapes, and higher analyte recoveries.
References
- Farsang, E.; Guillarme, D.; Veuthey, J. L.; Beck, A.; Lauber, M.; Schmudlach, A.; Fekete, S.,Coupling Non-denaturing Chromatography to Mass Spectrometry for the Characterization of Monoclonal Antibodies and Related Products. J Pharm Biomed Anal 2020, 185, 113207.
- Murisier, A.; Duivelshof, B. L.; Fekete, S.; Bourquin, J.; Schmudlach, A.; Lauber, M. A.; Nguyen, J. M.; Beck, A.; Guillarme, D.; D'Atri, V., Towards a Simple On-Line Coupling of Ion Exchange Chromatography and Native Mass Spectrometry for the Detailed Characterization of Monoclonal Antibodies. Journal of chromatography. A 2021, 1655, 462499.
- Chen, B.; Peng, Y.; Valeja, S. G.; Xiu, L.; Alpert, A. J.; Ge, Y., Online Hydrophobic Interaction Chromatography-Mass Spectrometry for Top-Down Proteomics. Analytical chemistry 2016, 88 (3), 1885–91.
- Lauber, M. A., Wanted: Native Protein LC-MS. The Analytical Scientist 2019.
- Goyon, A.; D'Atri, V.; Colas, O.; Fekete, S.; Beck, A.; Guillarme, D., Characterization of 30 Therapeutic Antibodies and Related Products by Size Exclusion Chromatography: Feasibility Assessment for Future Mass Spectrometry Hyphenation. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 2017, 1065–1066, 35–43.
- Shion, H.; Yu, Y.Q.; Chen, W.;A Platform Method for the Molecular Mass Analysis of the Light Chains and Heavy Chains of Monoclonal Antibodies using the BioAccord System. Waters Application Note 720006529EN.
720007455, December 2021