Determination of Pesticide Residues in Cottage Pie Baby Food Using GC-MS/MS With APGC™ After Extraction Using QuEChERS and Clean-up With Oasis™ PRiME HLB SPE
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
This application brief describes the validation of a comprehensive method based on gas chromatography-tandem mass spectrometry (GC–MS/MS) for the determination of over 200 pesticides in a typical baby food. Extracts of a cottage pie baby food were prepared using a modification of the QuEChERS CEN Method 156624, whereby the dispersive solid-phase extraction (dSPE) step was replaced with pass through SPE with Oasis PRiME HLB. The use of GC-MS/MS utilizing atmospheric pressure gas chromatography (APGC) has been shown to offer significant improvements in performance over electron ionization (EI) for pesticide residue analysis, in terms of selectivity and specificity. The extremely high sensitivity of the APGC Xevo™ TQ-XS System was demonstrated with reliable detection for almost all the analytes at concentrations as low as 0.00025 mg/kg, even when the injection volume was 1 µL. The method was successfully validated using the SANTE guidelines. The results from analysis of the spikes at 0.0005 and 0.001 mg/kg showed 87% and 93% of the analytes were within the required tolerance for recovery and 97% and 99.5% for repeatability. Pass through SPE with Oasis PRiME HLB offers a quick and effective alternative to dSPE. The method is considered sensitive, specific, accurate, and suitable for the determination of residues of a wide range of GC-amenable pesticides in baby food, for checking compliance with the specific MRLs set for food intended for infants and young children in Europe.
Benefits
- Pass-through clean-up with the Oasis PRiME HLB cartridge is an effective and quick means for removal of fats, phospholipids, and pigments from QuEChERS extracts whilst maintaining excellent recoveries for the pesticides of interest
- The APGC system is extremely sensitive and helps meet the needs of those involved with the analysis of baby foods for pesticide residues
- Very high sensitivity was achieved using splitless injection of 1 µL of an acetonitrile extract
Introduction
Infants and young children are considered as a vulnerable group when it comes to exposure to pesticide residues in foods. Maximum residue levels (MRLs) or tolerances are established in raw agricultural commodities and, in Europe, specific MRLs have been set for food intended for infants and young children. Following the precautionary principle, the legal limits for these types of food products were set at very low levels. In general, the default MRL of 0.01 mg/kg is applicable but more severe limitations were set for the most toxic pesticides and associated metabolites.1,2
Reliable analytical methods are needed for detection, quantification, and identification of hundreds of pesticide residues in food intended for infants and young children to check for compliance with these MRLs and by the food industry for due diligence and brand protection. We previously reported on the performance a method based on GC-MS/MS with APGC after the application of a conventional QuEChERS methodology for the analysis of pesticide residues in a processed cereal-based baby food.3 The method successfully utilized the enhanced sensitivity and selectivity provided by APGC to reliably quantity residues down to concentrations well below the MRLs specified for food for infants and young children.
The objective of this study was to demonstrate the performance of a method for the determination of pesticide residues in a more complex matrix – a processed baby food containing beef and various vegetables. Due to the higher fat content, the dSPE step was replaced by a pass through SPE clean-up step using Oasis PRiME HLB as this procedure is highly effective for removal of fats, phospholipids, and pigments from food extracts.4
Experimental
Sample Preparation, Extraction and Clean-up
Samples of cottage pie baby food were purchased from a local retail store and stored frozen until required. Samples were extracted using a modification of the QuEChERS CEN Method 15662,5 whereby the dSPE step was replaced by-pass through SPE with Oasis PRiME HLB in the Short Plus format. An overview of the details of the sample preparation and clean-up procedure used is given in Figure 1.
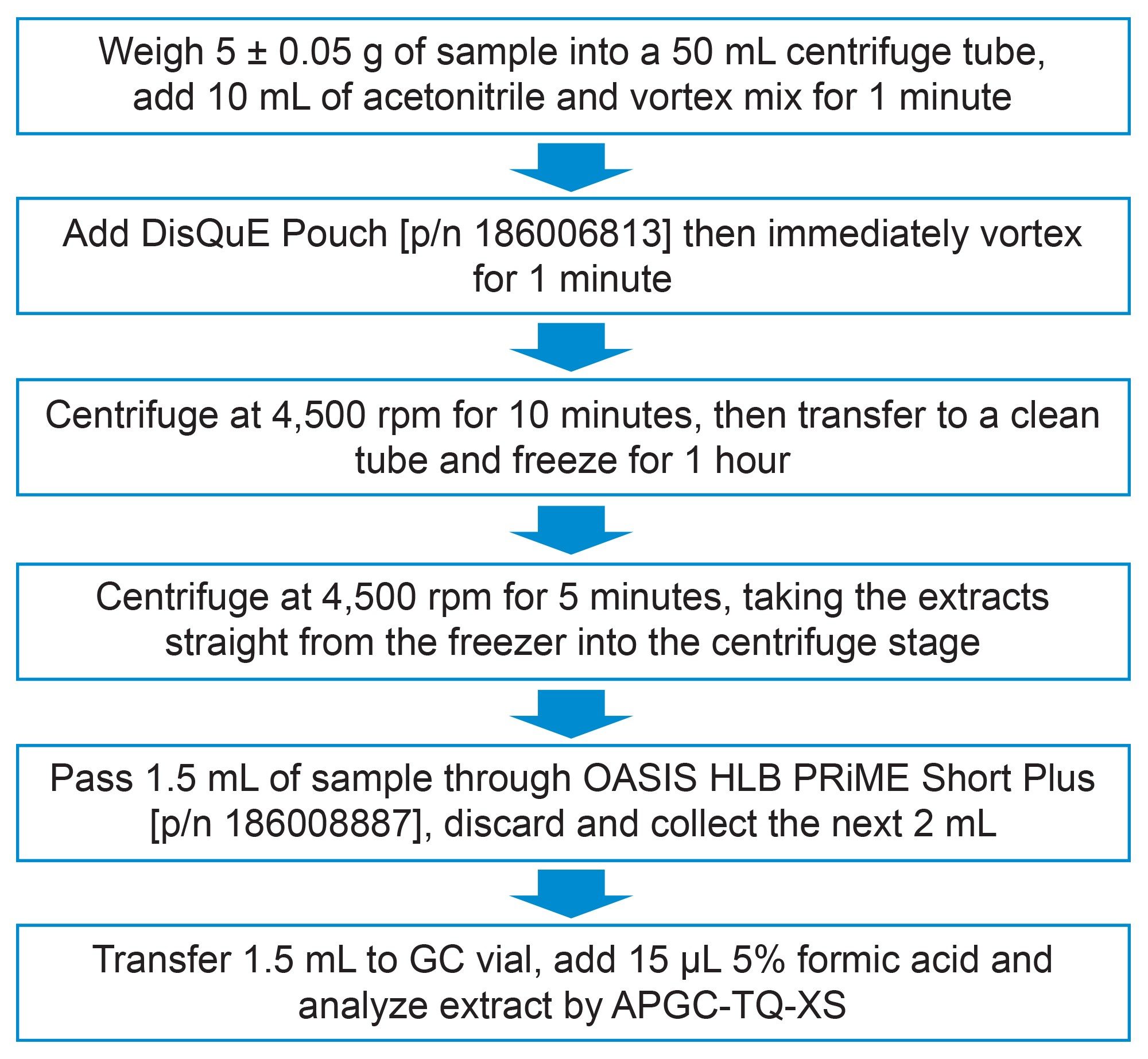
The GC Multiresidue Pesticide Kit (Restek p/n: 32562) was used to prepare working solution to create matrix-matched calibration standards and for spiking the baby food test portions. The calibration standards were prepared over the range 0.00025 to 0.02 mg/kg.
Details of the analytes, GC-MS/MS conditions and the validation protocol can be found in the previous application note.6 Recovery and repeatability were determined from the analysis of five replicates prepared at two levels: 0.0005 and 0.001 mg/kg.
Results and Discussion
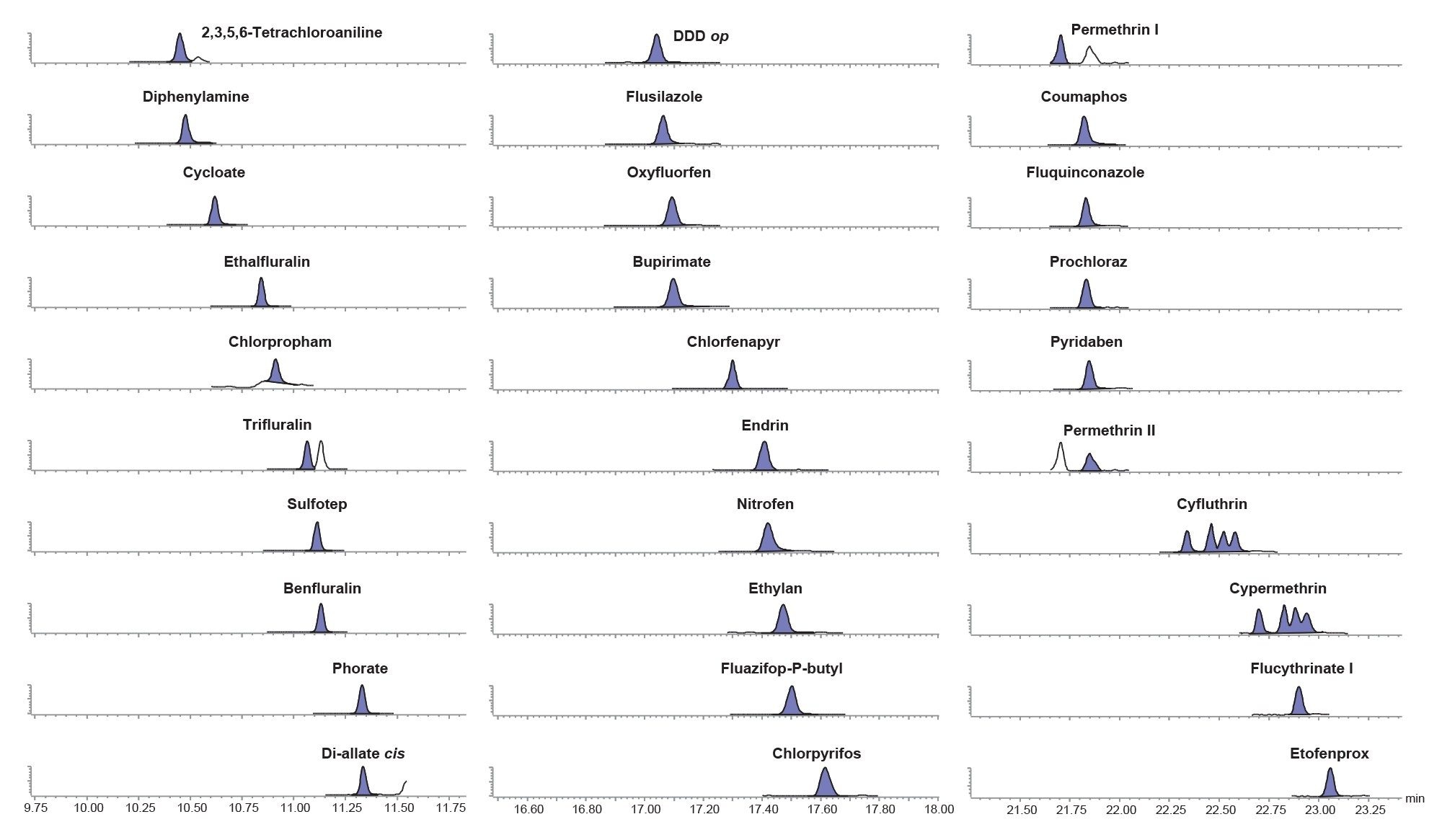
The sensitivity of the method was evaluated by assessment of the response of the matrix-matched standard at the lowest concentration prepared (0.00025 mg/kg; 0.25 µg/kg) and consideration of the response from the blank, which should be ≤30 % of the required reporting limit. Of the 211 analytes in the method, one was not detected (acequinocyl) and sensitivity for 2-phenylphenol, anthraquinone, biphenyl, and 2,3,5,6-tetrachloroaniline were all compromised by the presence of residues in the blank. From the remaining analytes, all but bioallethrin could be detected at 0.00025 mg/kg. Figure 2 show chromatograms from the analysis of a selection of pesticides in the baby food matrix-matched standard at 0.00025 mg/kg.
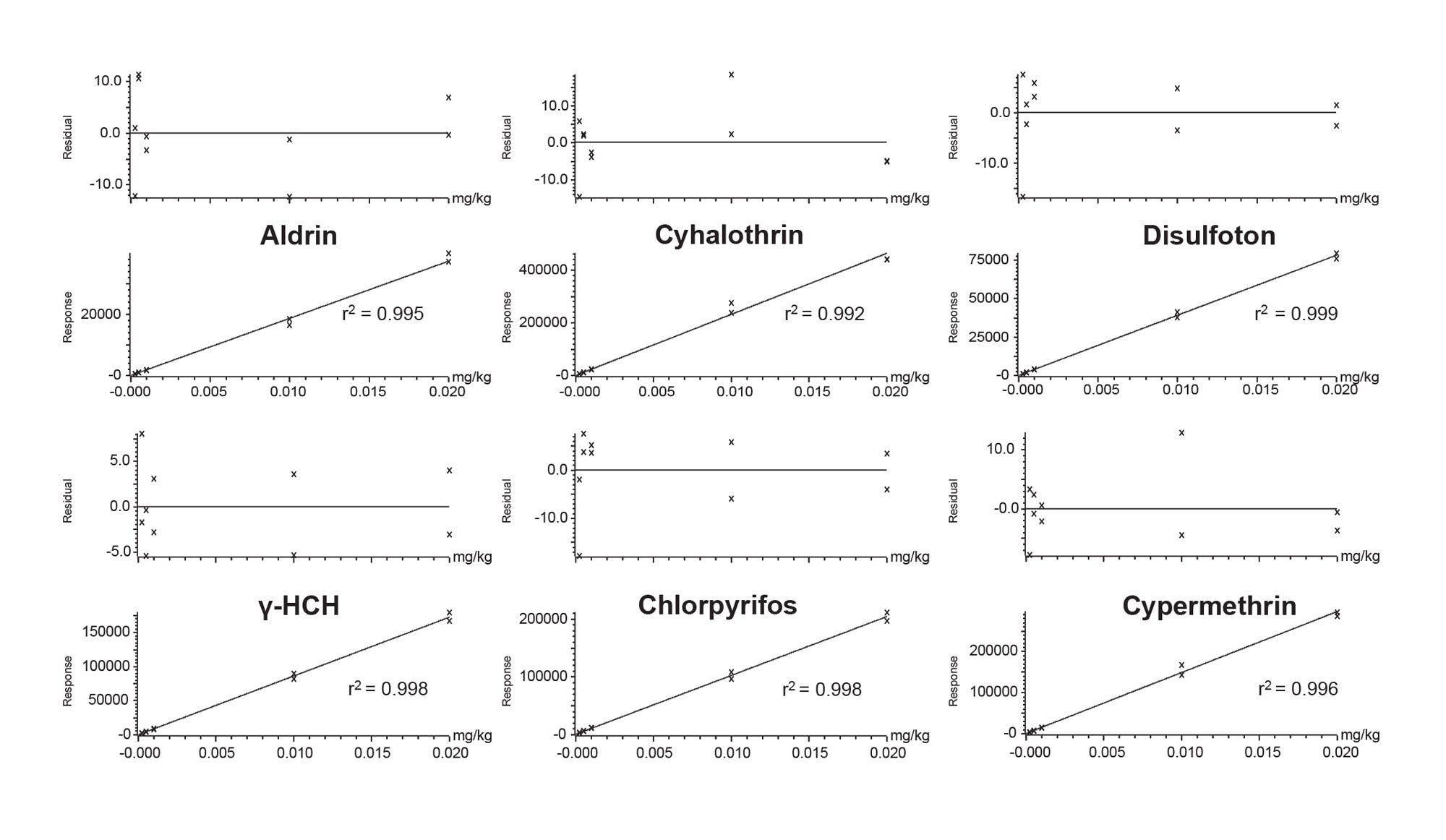
The deviation of the back-calculated concentrations of the calibration standards from the true concentrations (residuals), using the calibration curve in the relevant region should not be more than ±20%.7 97% of the analytes exhibited residuals well within the SANTE tolerance. Other than captan, edifenphos and folpet (r2 = 0.98), the graphs for all other analytes had values for r2 >0.99. Bracketed calibration graphs from the analysis of a selection of pesticides in baby food matrix-matched standards are given in Figure 3.
Identification criteria, retention times and ion ratios, were calculated and flagged using TargetLynx™. The retention time and ion ratio of each analyte detected in each spiked sample should correspond to that of the calibration standard reference. The retention times of all the analytes were found to be within the tolerance of ±0.1 minute. The ion ratios from the analysis of all the spiked samples were within ±30% of the average of calibration standards from same sequence for 92% of the analytes.
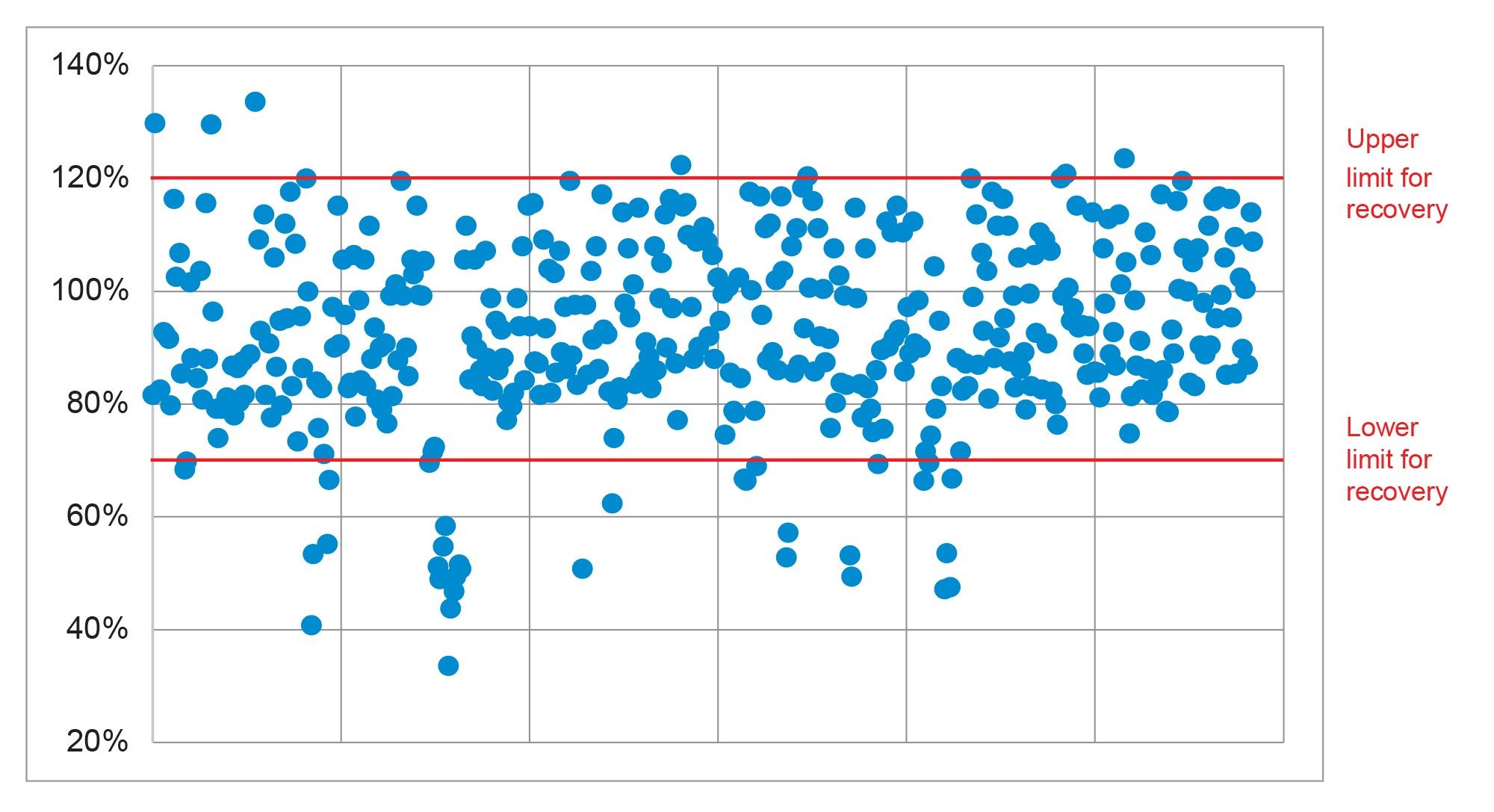
The recovery was evaluated using the data from the analysis of the five replicate spikes, at 0.0005 and at 0.001 mg/kg. The SANTE guidelines specifies an average recovery for each spike level tested to be between 70 and 120%. The results from analysis of the spikes at 0.0005 and 0.001 showed that 87 and 93% of the analytes were within that tolerance, respectively. Eight compounds were either not detected at 0.0005 mg/kg or quantitation compromised by the presence of compounds in the bank. The remaining compounds at both concentrations all exhibited recoveries between 30% and 140%, but they were consistent (RSD ≤20%). A summary of all the recovery results is shown in Figure 4.
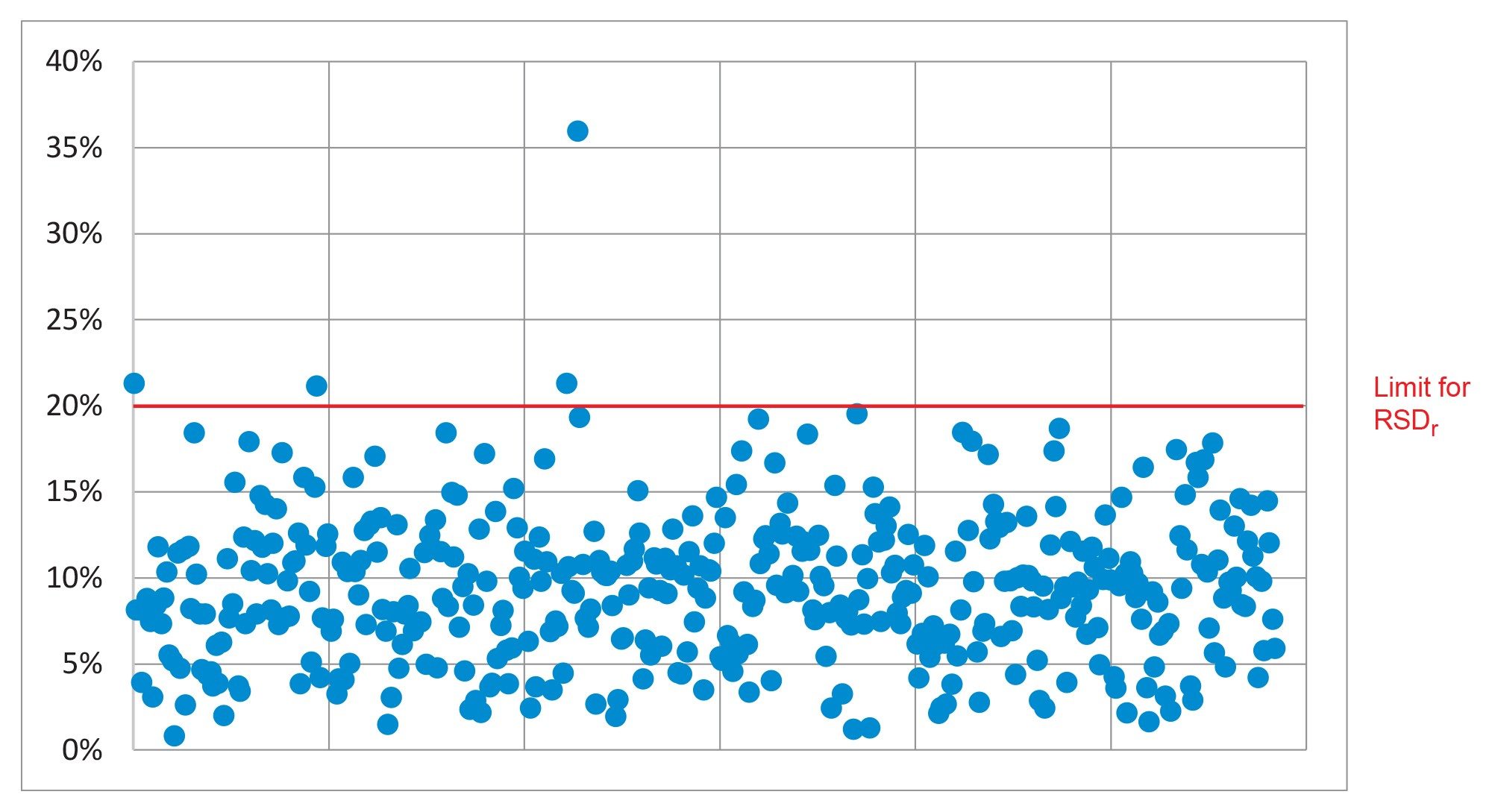
The repeatability (RSDr) of the method was also satisfactory. SANTE guidelines states that RSDr for each spike level tested should be ≤20%. At 0.0005 and 0.001 mg/kg, 97 and 99.5% of the analytes were within this tolerance, respectively. A summary of all the repeatability results is shown in Figure 5.
Conclusion
This application brief describes a sensitive and accurate multiresidue method for the determination of pesticide residues in cottage pie baby food using GC-MS/MS (Xevo TQ-XS fitted with APGC). The method allowed for reliable quantitation down to concentrations well below typical MRLs and was successfully validated according to the SANTE guidelines. The method exhibited very high sensitivity with reliable detection for all the analytes at very low concentrations even when the injection volume was 1 µL. Pass through SPE with Oasis PRiME HLB offers a quick and effective alternative to dSPE. This method has been demonstrated as suitable for monitoring for the presence of pesticide residues at very low levels in baby food including checking compliance with the specific MRLs set for food intended for infants and young children in Europe.
References
- COMMISSION DELEGATED REGULATION (EU) 2021/1040 of 16 April 2021 Amending Delegated Regulation (EU) 2016/128 as Regards the Requirements on Pesticides in Food for Special Medical Purposes Developed to Satisfy the Nutritional Requirements of Infants and Young Children. https://eur-lex.europa.eu/legal-content/DE/ALL/?uri=CELEX:32021R1040.
- COMMISSION DELEGATED REGULATION (EU) 2021/1041 of 16 April 2021 Amending Delegated Regulation (EU) 2016/127 as Regards the Requirements on Pesticides in Infant Formula and Follow-on Formula. https://eur-lex.europa.eu/legal-content/DE/ALL/?uri=CELEX:32021R1041.
- Determination of Pesticide Residues in Rice-Based Baby Food Using GC-MS/MS With APGC™ After Extraction and Clean-up Using QuEChERS. Waters Application Brief 720007682.
- Oasis PRiME HLB Food Applications Notebook. Waters Applications Notebook 720005932.
- European Committee for Standardisation (CEN) EN 15662:2018. Foods of Plant Origin - Multimethod for the Determination of Pesticide Residues Using GC- and LC- Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up By Dispersive SPE - Modular QuEChERS-Method.
- Determination of Pesticide Residues in Cucumber Using GC-MS/MS With APGC™ After Extraction and Clean-up Using QuEChERS. Waters Application Note 720007654.
- Document No. SANTE/11312/2021. Guidance Document on Analytical Quality, Control, and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. 2021.
720007708, August 2022