Reliable Analysis of Aspirin and Related Substances in Drug Substance and Tablet Formulation Using an Alliance™ iS HPLC System
Abstract
Robust and reliable analytical test methods are essential to ensure the quality and safety of pharmaceutical drug products. This work describes a single high performance liquid chromatography (HPLC) method for the analysis of Aspirin active pharmaceutical ingredient (API) and six associated related substances. The analysis is performed on the Alliance iS HPLC System with the XSelect™ HSS T3 Column. The system suitability, linearity, accuracy, intraday, and interday method performance are assessed, generating excellent results while meeting the USP requirements (USP43-NF38, Aspirin Tablets). This work also demonstrates applicability of the method for a reliable and accurate determination of aspirin assay and related substances content in drug substance and tablet formulation.
Benefits
- Reliable and quick method (within 7.6 mins) for the simultaneous determination of aspirin assay and related compounds (impurities) in the drug substance and tablet formulation
- Precise, repeatable, linear, accurate, and excellent intraday, and interday method performance demonstrated on the Alliance iS HPLC System
Introduction
To ensure compliance with the current good manufacturing practices (CGMP) regulations, pharmaceutical manufacturers must demonstrate the identity, strength, quality, and purity of drug products.1 This requires the establishment of robust analytical test methods that produce reliable and accurate results during routine use in the quality control (QC) laboratory.
Aspirin is a commonly used drug for relieving minor aches, pains, and fevers, as well as the prevention of heart attacks and mini strokes. Aspirin is available in tablets for oral administration, with 81–650 mg of aspirin per tablet.2 One method was found in the literature for the simultaneous separation of aspirin and its six related substances, described in the European Pharmacopeia for Acetylsalicyclic Acid.3 The United States Pharmacopeia (USP) monograph for aspirin tablets only specifies one impurity in the procedure.4
Herein, a method for the analysis of aspirin API and associated related substances was developed and aspirin samples analyzed on an Alliance iS HPLC System. The critical method performance characteristics including system suitability, linearity, accuracy, intraday, and interday performance were assessed during the study. The results were analyzed and compared against the acceptance criteria listed in the USP monograph for Aspirin Tablets.4 The method exhibited excellent intraday and interday performance, with relative standard deviations (RSD) of peak areas and retention times of ≤0.25% and ≤0.03%, meeting the USP criteria. Additionally, the method was shown to be suitable for aspirin assay and related substances (impurities) content determinations in the drug substance and tablet formulation.
Experimental
Standard Solutions
Individual stock standard solutions with related substances and aspirin API were prepared in diluent (60:40 water/acetonitrile with 0.1% formic acid) at 1.0 and 5.0 mg/mL, respectively. The API stock solution was diluted with diluent to 0.1 mg/mL and spiked with related substances at the 10% level to make a mixture standard solution. Aspirin and six related substances specified by the European Pharmacopeia are listed in Table 1.3
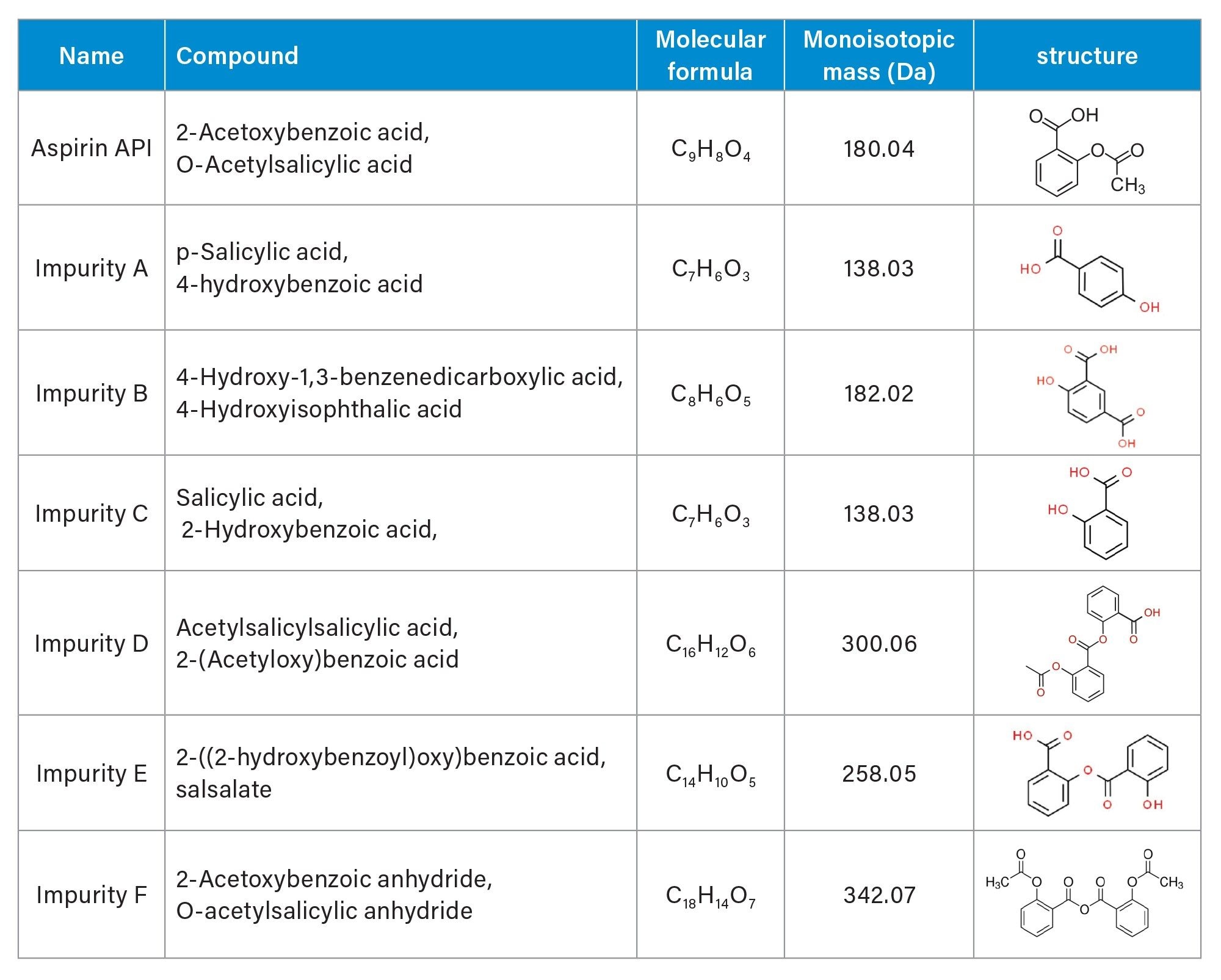
Aspirin Sample Solutions
Crushed tablets (containing 81-mg of aspirin) were dissolved in diluent (60:40 water/acetonitrile with 0.1% formic acid) at 1.6 mg/mL of aspirin by sonication for ten minutes. After extraction, sample test solutions were centrifuged for ten minutes at 3000 rpm and diluted to 0.1 mg/mL for aspirin assay. For related substances testing, the drug tablets and drug substances were prepared at 0.5 mg/mL in diluent (60:40 water/acetonitrile with 0.1% formic acid).
Solutions were filtered through a 0.2 µm Nylon syringe (Waters p/n: WAT200524) filter prior to analysis.
Conditions
|
LC system: |
Alliance iS HPLC System with TUV detector |
|
Vials: |
LCMS Maximum Recovery 2 mL, p/n: 600000749CV |
|
Column(s): |
XSelect HSS T3, 4.6 x 150 mm, 3.5 µm (p/n: 186004786) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
15 µL |
|
Mobile phase: |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
|
Wash solvents: |
Purge/Sample Wash: 60:40 water/acetonitrile Seal Wash: 90:10 water/acetonitrile |
|
Detection: |
237 nm |
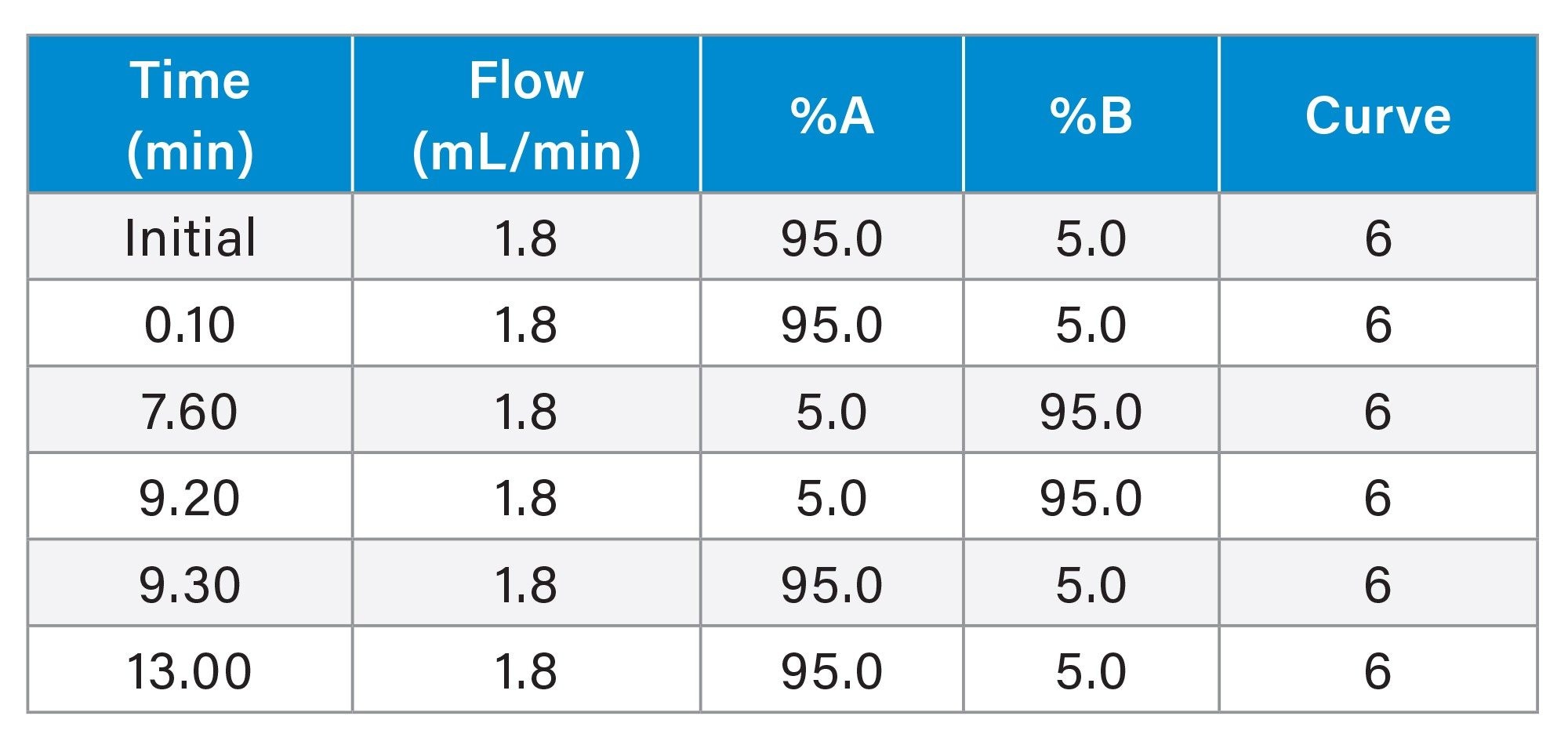
Gradient Table
Software
|
Chromatography data software (CDS): |
Empower™ 3.6.1 |
Data acquisition and analysis performed using Empower Software. Summary reports generated using the report templates available in the Empower project.
Results and Discussion
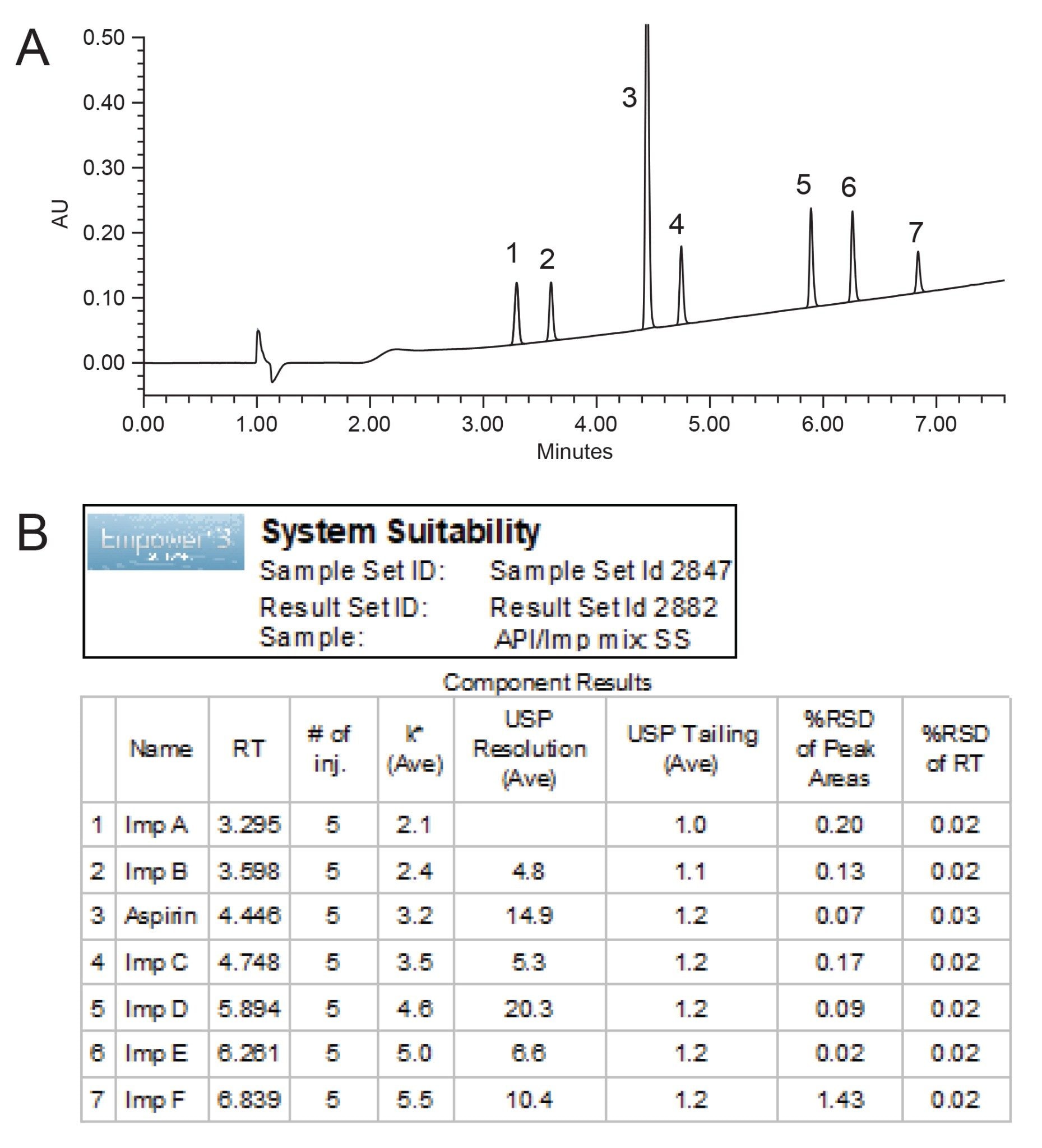
The developed method was run on the Alliance HPLC iS System and successfully separated aspirin API and six associated related substances within 7.6 minutes, with a minimum USP Resolution (USP Rs) ≥4.8, peak tailing of 1.0–1.2, and retentivity factor (k*) ≥2.1 (Figure 1).
System Suitability
System suitability was assessed using five replicate injections of the standard mixture solution, assuring the suitability requirements specified in the USP monograph for aspirin tablets were met. The USP suitability requirements listed for the assay and impurities procedures include4:
- Assay: peak tailing of not more than (NMT) 2.0 and relative standard deviation (RSD) of NMT 2.0
- Impurities: resolution between salicyclic acid and aspirin peaks of not less than (NLT) 2.0; RSD for salicyclic acid of NMT 4.0%
The method met the system suitability requirements, with the RSD for peak areas and retention time (RT) ranging from 0.07 to 1.43% and 0.02 to 0.03%, respectively (Figure 1B). Additionally, the USP suitability requirements for the assay and impurities procedures were met.
Intraday and Interday Performance
Demonstrating intraday and interday performance ensures that the method run on the Alliance iS HPLC generates consistent results, which is critical to the quality control and safety of the pharmaceutical products.
The intraday method performance on the Alliance iS HPLC System was evaluated by analyzing five replicate injections of the mixture standard solution in four different sets in a one-day analysis. The results were assessed against the USP suitability requirements for Aspirin Tablets.4 The intraday method performance was excellent, meeting the USP suitability requirements defined for aspirin assay and impurities procedures (Table 2). The RSD of aspirin peak areas and retention times ranged from 0.03% to 0.08% and 0.02 to 0.03% over four sets of data. The RSD for salicyclic acid (impurity C) peak areas ranged from 0.07 to 0.25%.
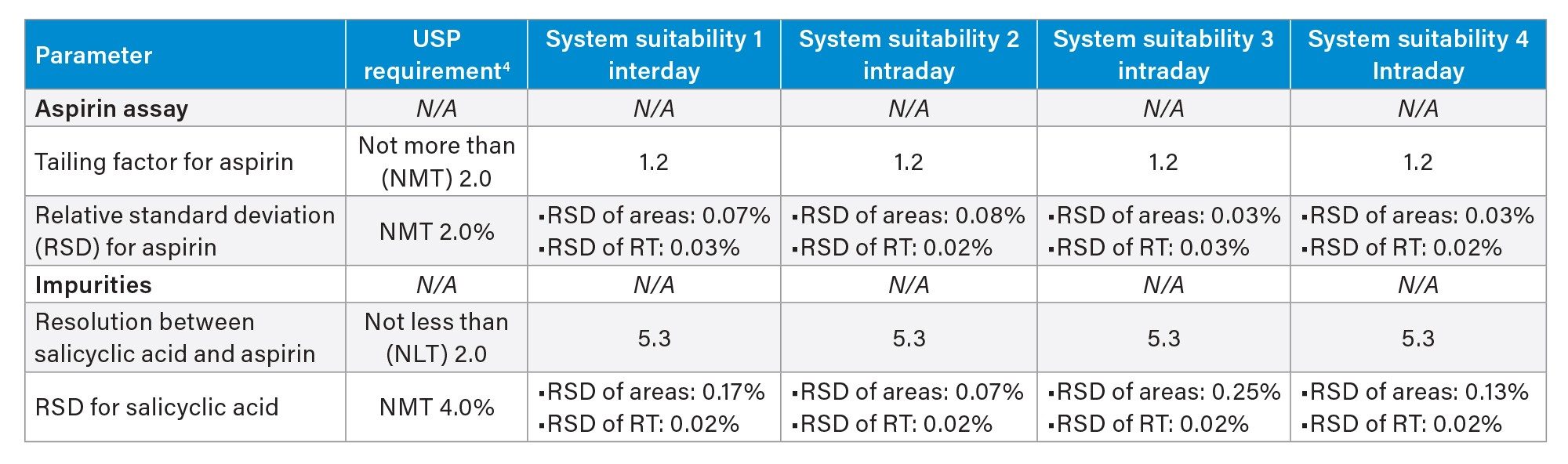
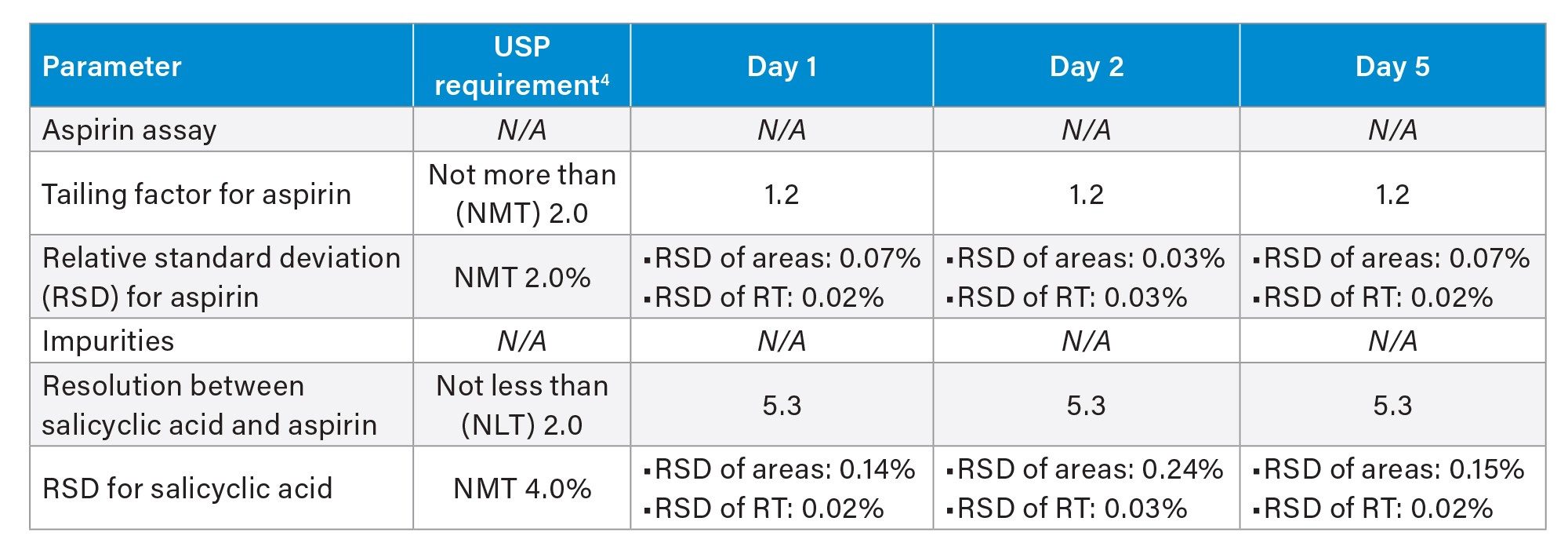
For interday performance of the method run on the Alliance iS HPLC System, a mixture standard solution was analyzed over different days including day 1, 2, and 5. The method exhibited excellent performance, generating comparable results across the tested days and meeting all the USP suitability criteria (Table 3).
Linearity and Range
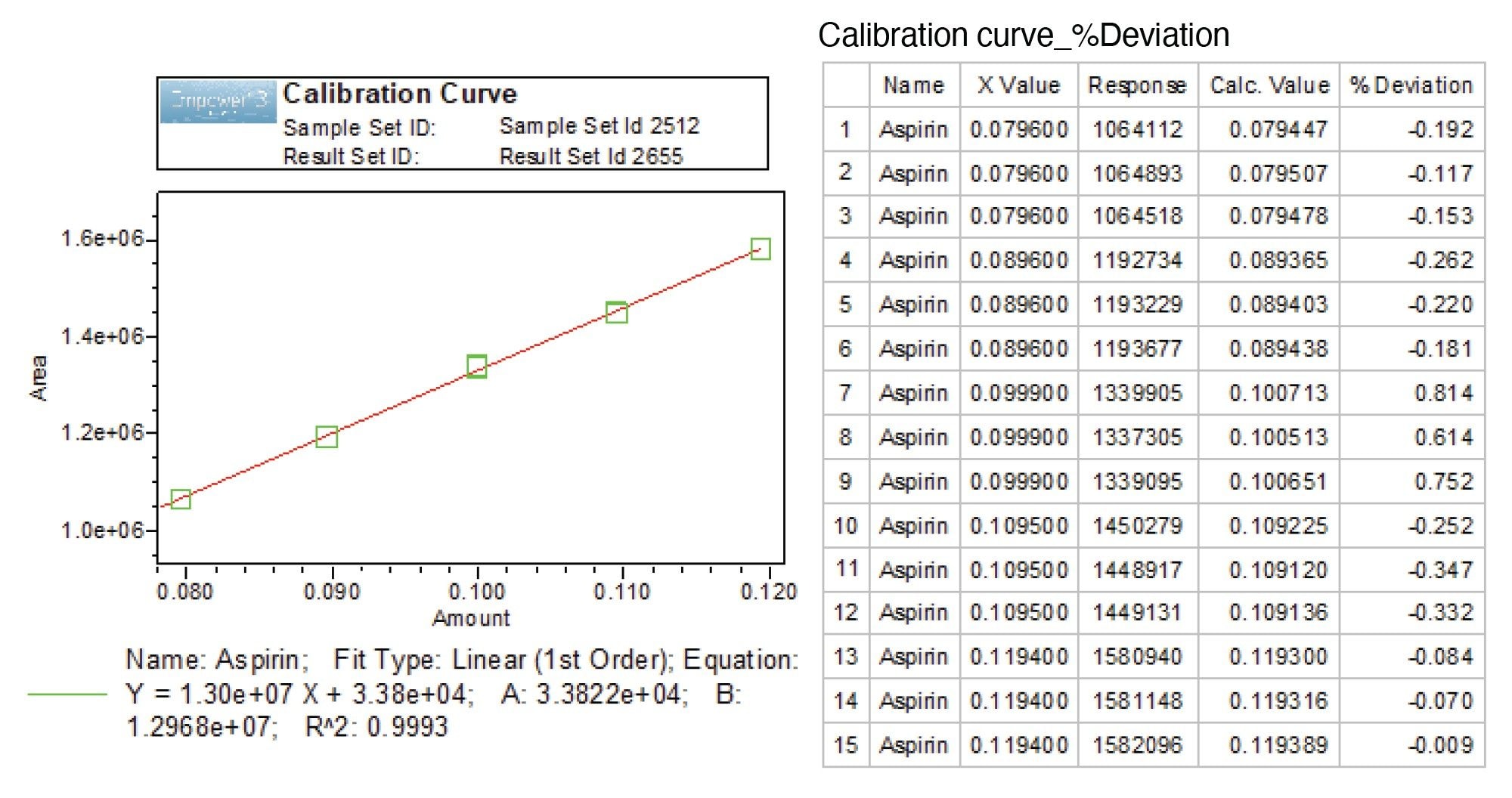
The calibration plot for aspirin API was constructed in the range of 80 to 120% with respect to the API working concentration in the sample preparation of 0.1 mg/mL. The calibration curve of the concentration versus the peak area at each level produced a correlation coefficient (R2) ≥0.999 (Figure 2). In addition, the percent deviation of the calculated X values or concentrations ranged from –0.35 to 0.81%.
Aspirin Assay
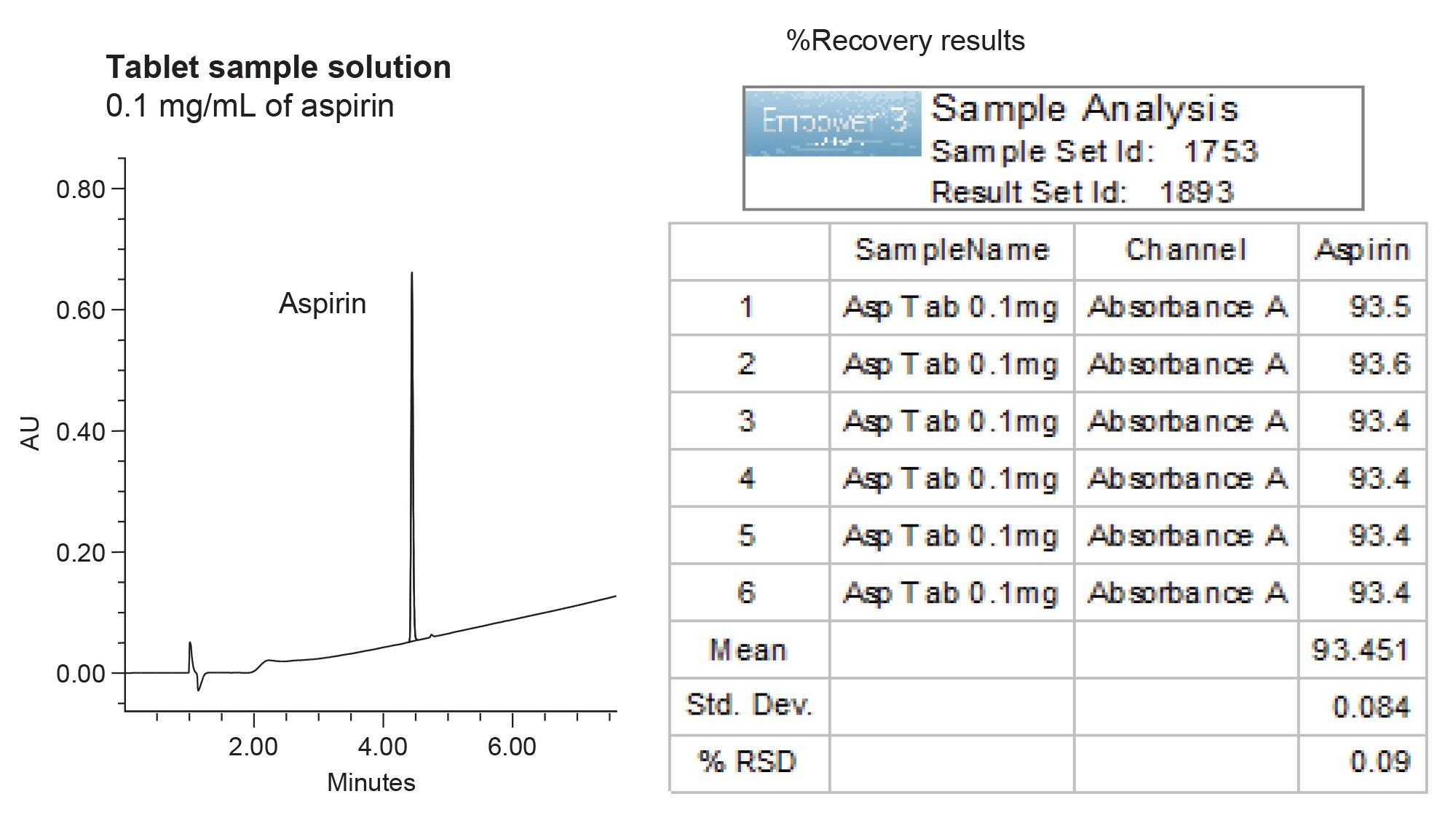
For assay testing, the drug tablets containing 81-mg of aspirin were prepared at 0.1 mg/mL in diluent (60:40 water/acetonitrile with 0.1% formic acid). To calculate % recovery, the sample solutions were quantified against the calibration curve. The assay results for six samples ranged from 93.4 to 93.6% (Figure 3), meeting the USP acceptance criteria of not less than (NLT) 90.0 and not more than (NMT) 110.0% of the labeled amount of aspirin.4
Related Substances
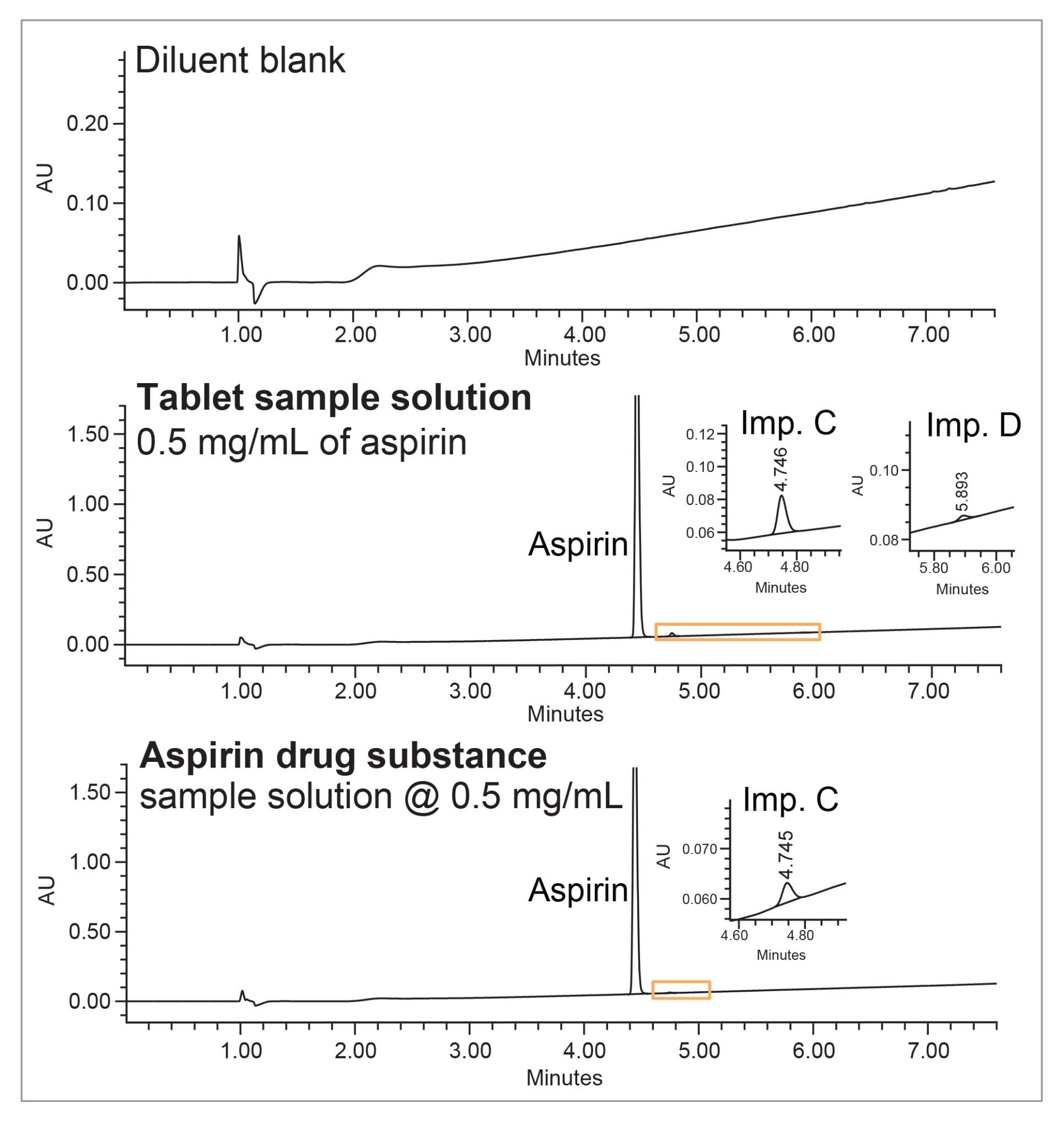
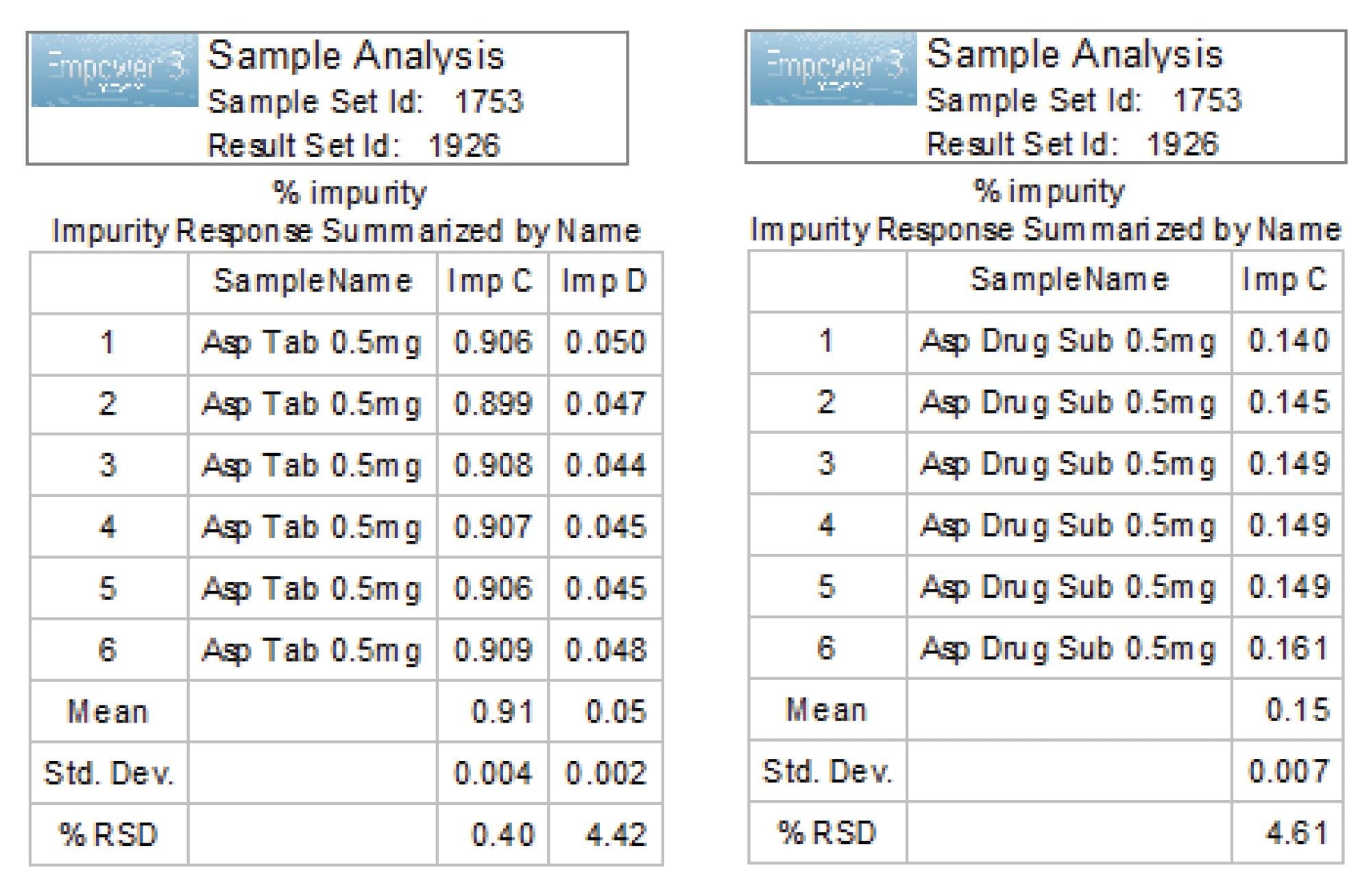
The related substances content (% impurity) was determined by comparing peak areas of each related substance to the aspirin API peak area (Figure 4).
The analysis revealed presence of two impurities in the aspirin tablet samples (impurity C and D at 0.91% and 0.05%) and presence of impurity C at 0.15% level in the drug substance sample (Figure 5). Results met the USP acceptance criteria for salicyclic acid (imp. C): NMT 0.3% and for coated tablets of NMT 3.0%.4
Conclusion
A single LC method for aspirin and all six related substances was developed and run on the Alliance iS HPLC System, demonstrating excellent performance suitable for the simultaneous determination of aspirin API, and six associated related substances (impurities) content in the drug substance and tablet formulations. System suitability results met the criteria for aspirin assay and impurities procedure. Excellent linearity, accuracy, and intraday and interday performance have been demonstrated. Results demonstrate that the Alliance iS HPLC delivers robust, reliable, and reproducible performance suitable for routine analytical testing.
References
- Current Good Manufacturing Practice (CGMP) Regulations | FDA.
- https://medlineplus.gov/druginfo/meds/a682878.html
- Ph. Eur. Monograph. Acetylsalicyclic Acid. The European Pharmacopeia 10.0. 01/2017:0309.
- USP monograph for Aspirin Tablets. United States Pharmacopeia USP 43-NF 38, official 1st May 2020.
720007994, August 2023