
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates about Waters Automated Method Development System (AMDS) which provides tools to effortlessly transfer a method between instruments.
Traditional manual method development and transfer can be a labor-intensive, time-consuming, and often imprecise process. The process becomes even slower and more frustrating when the method that needs to be transferred requires modification in the end user’s lab. The Waters Automated Method Development System (AMDS) is a tool that can increase efficiency and throughput to streamline these issues.
As technology advances, more and more labs are upgrading equipment. Unfortunately, when an instrument is upgraded, quite often the method performs differently than it did on the older system. Retention times don’t match, and sometimes the chromatographic separation or resolution suffers.
The first step in successfully transferring a method is to gather information about the new and existing system. One critical piece of information is obtained by measuring dwell volume, also known as the gradient delay volume (See Figure 1). Dwell volumes are often described as small values to promote the quality of the system for marketing purposes.
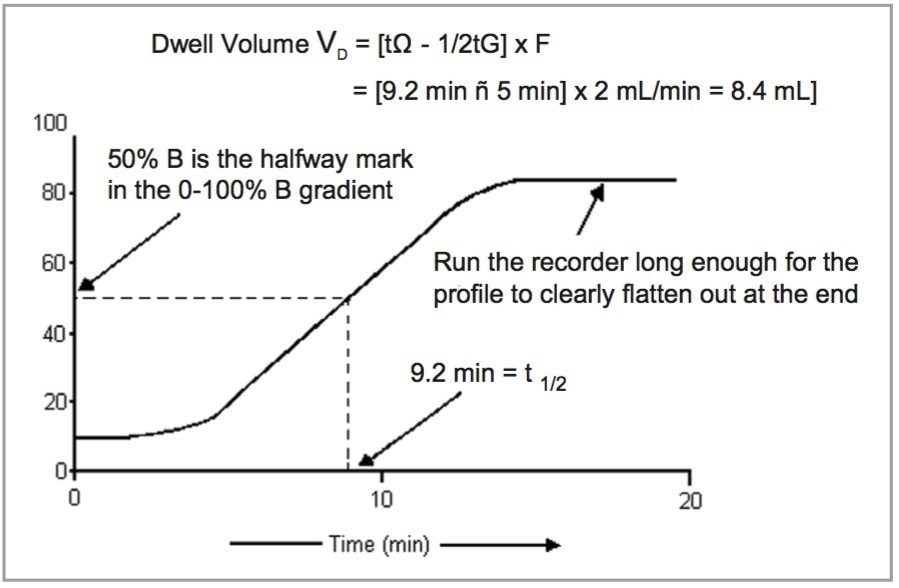
However, many times vendors are not actually showing the chromatographic sense of dwell volumes, the actual point of homogenous gradient mixing and lift-off point of a gradient curve. These volumes are generally greater.
Older HPLC systems generally have greater dwell volumes than today’s modern technology and can range from 2-6 mL. In newer instruments, the volume range can be 0.5-1.3 mL and less.
Method transfer can be as easy as 1-2-3 Using the method transfer capabilities of the Waters AMDS, nearly identical results can be obtained from different HPLC dwell volumes for a seamless transfer between instruments.
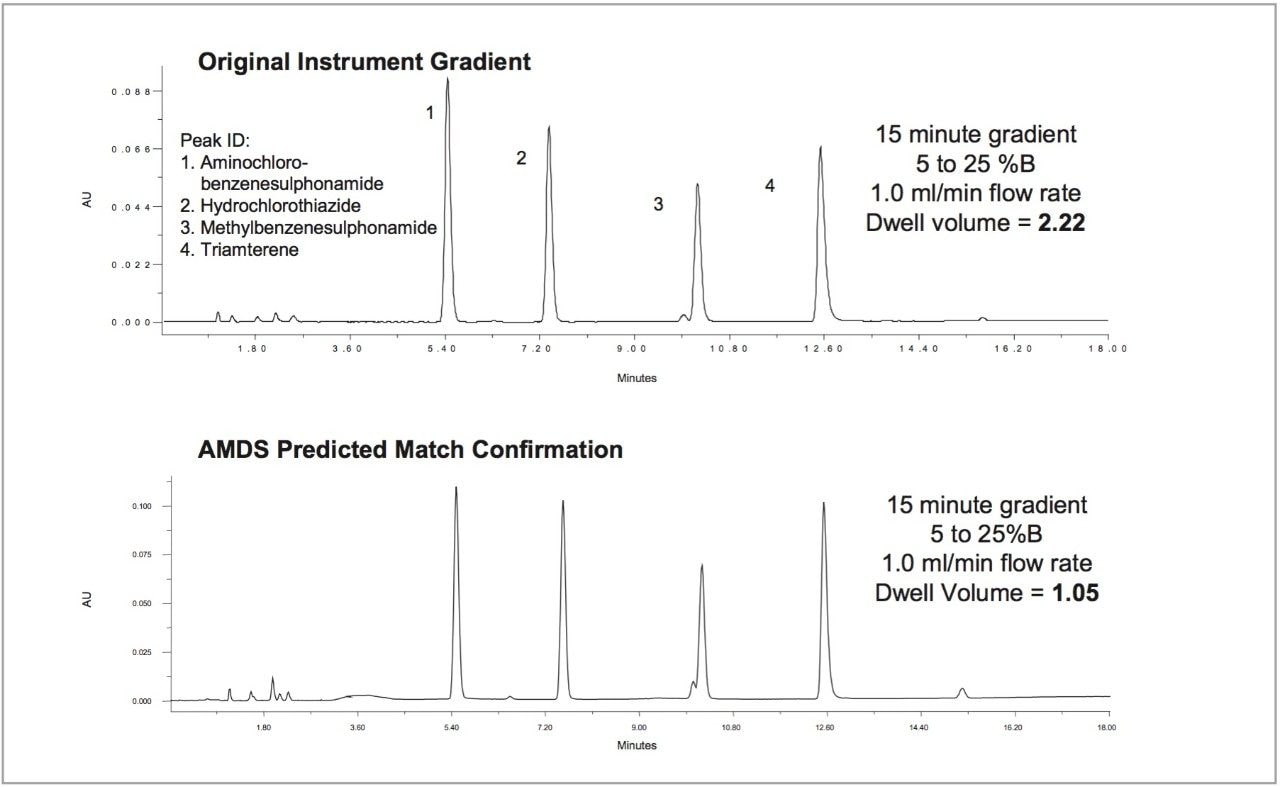
In Figure 2, the original method was developed on a system with a dwell volume equal to 2.22 mL. The Waters Alliance System to which it was transferred measured a dwell volume equal to 1.05 mL. Once the automated calibration experiments were performed and automatically entered into Drylab 2000plus, a chromatographic match was achieved by manipulating the Drylab Gradient Editor.
The following simple steps can help you transfer any method when using the original chromatogram as a reference:
In this example, a gradient hold at the beginning of the injection was sufficient enough to yield a matching chromatogram. In some cases, however, some further manipulations with the Resolution Plot may be needed to match chromatograms between systems with varying gradient proportioning valves and mixing performance.
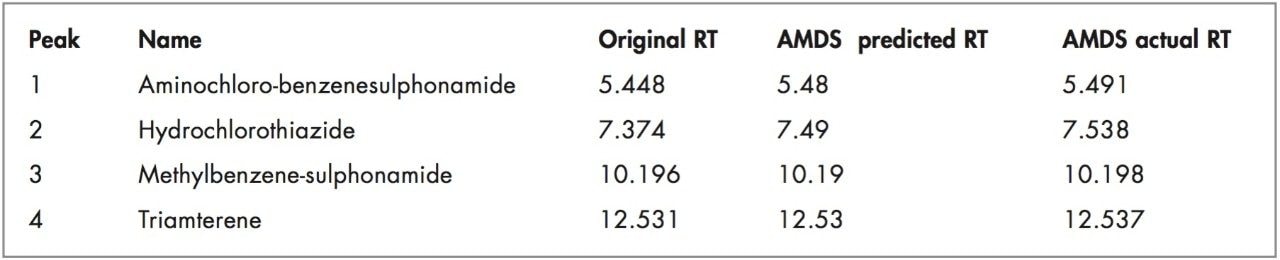
AMDS also accurately predicts retention times, resolution, solvent used, average k’ for any changes in column dimensions, solvent strength, temperature, or gradient modifications. Comparing actual to predicted results generally yields differences that can range from 0 to 0.8 minutes depending on the accuracy of the measured dwell volume, extracolumn volume, and integrated data (Figure 3).
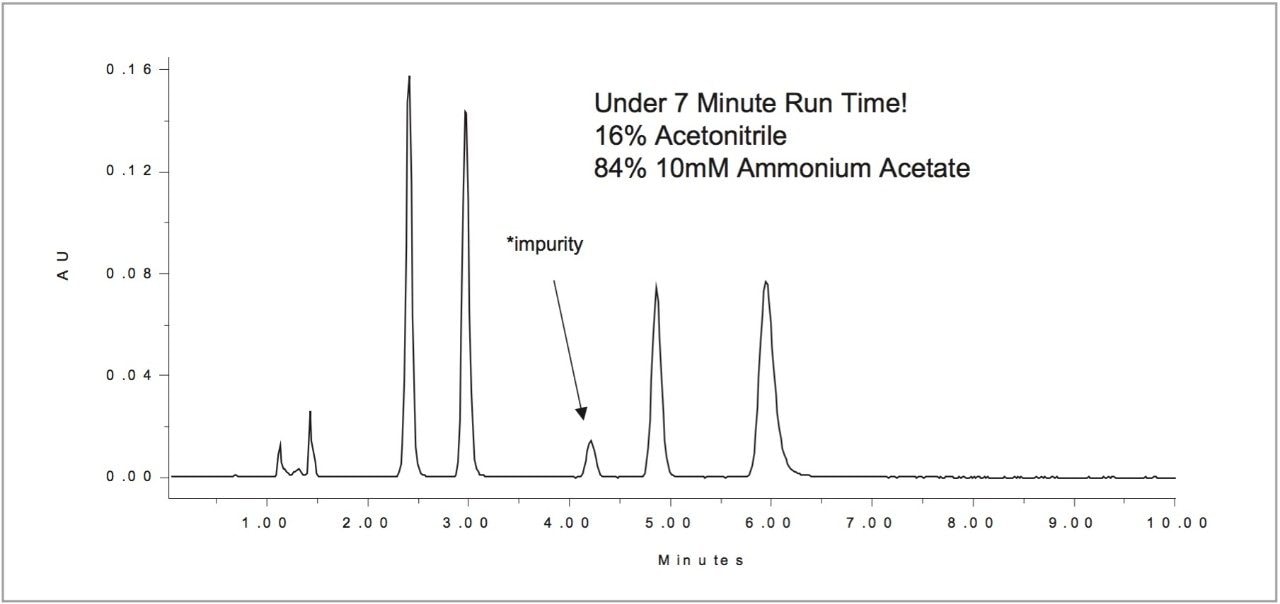
With the Waters Automated Method Development System, you can develop more robust and less problematic methods (See Figure 4). AMDS can perform an optimal LC chromatogram without the need for additional experiments. By selecting “continue analysis until complete” on the last setup wizard screen, you can optimize your method according to goals that you suggest. The end result not only provides the you with Drylab2000 simulation data, but also a method that may result in a faster, more robust, chromatogram.
In many cases, when upgrading to new lab equipment, methods are developed in haste due to time constraints. The Waters AMDS provides the tools necessary to effortlessly transfer a method between instruments.
Moving to newer technology is made easier once accurate dwell volume measurements are obtained. In a three-step process, you can obtain valuable chromatographic information — displayed colorfully in a Drylab resolution plot — that simulates changes using different variables, as well as providing indications of method robustness.
The Gradient Editor is likewise an excellent tool that can simulate mixing differences between instruments, simulating multiple gradients or gradient holds. Prediction accuracies are usually within ±1 minute and your end result is often a more optimized method.
The Waters AMDS is an excellent tool to transfer methods efficiently while ultimately saving time and money.
720000752, October 2003