
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the low carryover of the ACQUITY UPLC I-Class System, of particular importance for the bioanalysis of low-exposure medicines and during the dose escalation stage where compound concentration changes significantly during a study.
The low carryover characteristics of the ACQUITY UPLC I-Class System are critical to successfully resolve analytes such as fluticasone propionate that require an assay with sensitivity in the low pg/mL range.
The ability to accurately determine the pharmacokinetic fate of a new candidate drug molecule in a biological system often requires a very high sensitivity bioanalytical assay. By combining state-of-the-art sample preparation technology, high-resolution sub-2-µm UPLC chromatography, and modern tandem quadrupole mass spectrometers it is possible to achieve assays in picogram per millilitre range.
However, the level of sensitivity at which assays can be validated and reliably used can be compromised by carryover in an analytical system. The U.S. FDA guidelines for assay validation1 recommend that the peak area of a blank injection following the injection of the highest concentration standard should not exceed 20% of the peak area of the limit of quantification standard. Instrumentation with poor carryover characteristics can limit the assay range and also method applicability.
In LC-MS/MS methodologies, the observed carryover is usually attributed to the chromatographic autosampler. To minimize carryover, it is often necessary to perform several wash steps using a series of wash solvents. Identifying the appropriate wash solvents can take a significant amount of method development time and experimentation. Additionally, the addition of the wash solvent protocols adds 1 to 2 minutes to complete the injection process, reducing productivity and throughput of the assay.
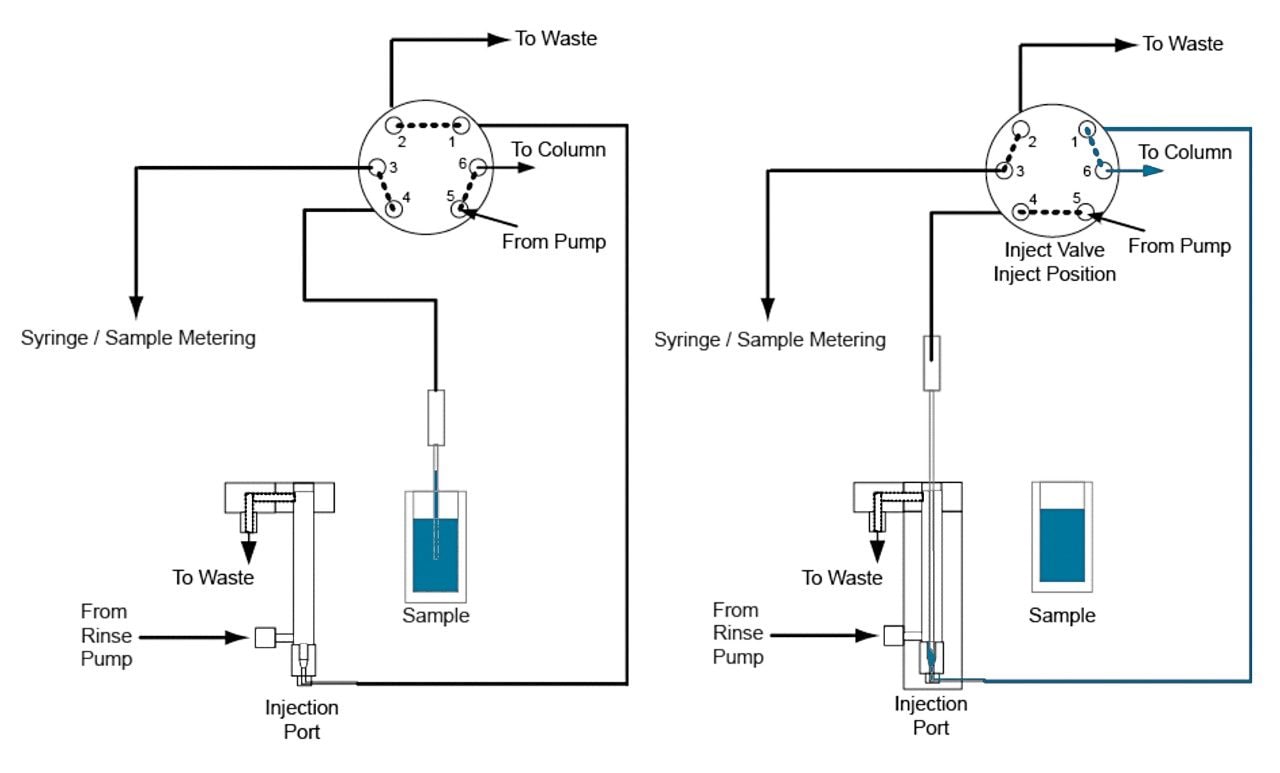
There are two main approaches for introducing the sample onto the chromatography system: via a fixed loop or a flow-through needle. The fixed-loop approach offers the lowest dispersion characteristics and minimal delay volume, while the flow-through needle design offers the greatest flexibility in injection volumes.
The ACQUITY UPLC I-Class System is the first binary chromatography system that delivers the flexibility and simplicity of a flow-through needle design with the chromatographic performance derived from ultra-low dispersion (Figure 1).
Fluticasone propionate is an inhaled corticosteroid used to treat asthma, chronic obstructive pulmonary disease, and allergic rhinitis (hay fever). Its sites of action are the lungs and airways. It also has very poor absorption characteristics and, as such, exhibits extremely low systemic exposure.
In order to accurately define the pharmacokinetics of fluticasone propionate, it is necessary to develop an assay with sensitivity in the low pg/mL range (1 to 5 pg/mL) with a dynamic range of three to four orders of magnitude; thus a low-carryover system is critical to allow an assay to be validated and employed at these low levels.
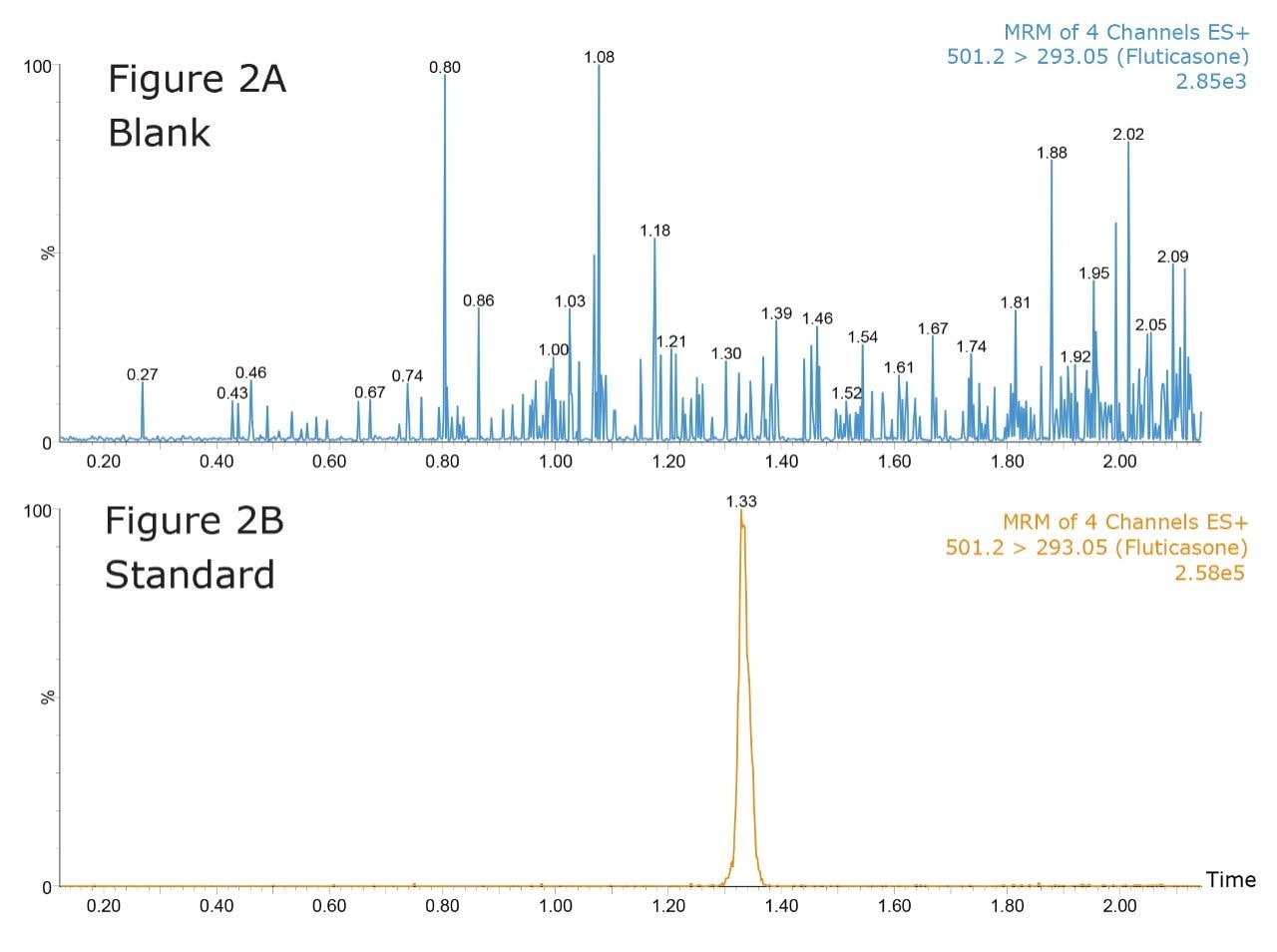
Figure 2 shows the UPLC-MS/MS analysis, using MRM mode, of a blank solvent sample following the injection of high-concentration standard at 5 ng/mL. The retention time of the fluticasone peak is 1.33 minutes. As we can see from the data in Figure 2A the system exhibits extremely low carryover characteristics with the fluticasone peak not visible in the blank injection at 1.33 minutes.
The ACQUITY UPLC I-Class System has been specifically designed to deliver optimal chromatographic performance in terms of band spreading and dispersion. Its new flow-through needle design provides:
720003938, May 2011