The BEH Amide bonded phase is more compatible with slightly higher aqueous content in mobile phases and sample diluents than unbonded HILIC stationary phases. In this application note, we demonstrate the utility of the BEH Amide particle for the analysis and isolation of a hydrophilic peptide.
BEH Amide columns are specifically designed to enhance the retention of polar compounds, making analysis, scaling, and isolation easier.
Reversed-phase columns are typically used for the analysis and isolation of peptides, however some hydrophilic peptides have little or no retention on C18 stationary phases. Insufficient interaction with the stationary phase leads to difficulties in the peptide isolation. Hydrophilic Interaction Chromatography (HILIC) is an alternative chromatographic technique useful in the isolation of compounds where analytes are separated based on a unique combination of liquid-liquid partitioning, adsorption, ionic interaction, and hydrophobic retention mechanisms. Compounds elute from the column as the gradient transitions from low aqueous to high aqueous mobile-phase composition.
The BEH Amide column, with a trifunctionally-bonded amide phase, was first introduced in 2009 with 1.7 μm particles for the analysis of polar compounds using the ACQUITY UPLC System. Demand for a column capable of analyzing compounds such as hydrophilic synthetic peptides, saccharides, synthetic sugars, glycopeptides, and polar compounds from natural products has driven the development of a larger 5 μm particle for use in analytical and preparative HPLC applications. In this application note, we demonstrate the utility of the BEH Amide particle for the analysis and isolation of a hydrophilic peptide.
|
System: |
Waters 2525 Binary Gradient Module, 2767 Sample Manager, Column Fluidics Organizer, 2996 Photodiode Array Detector, ZQ 2000 Mass Spectrometer, and 2420 ELSD Mass Detector |
|
Columns: |
XBridge BEH Amide, 5 μm, 4.6 x 150 mm, part number 186006595 XBridge BEH Amide, 5 μm, 19 x 150 mm, part number 186006605 |
|
Column Temp.: |
40 °C |
|
Mobile Phase A: |
20/80 acetonitrile/ 10 mM ammonium formate pH 3 |
|
Mobile Phase B: |
90/10 acetonitrile/ 10 mM ammonium formate pH 3 |
|
Weak Needle Wash: |
90/10 acetonitrile/water |
|
Strong Needle Wash: |
20/80 acetonitrile/water |
|
Seal Wash: |
50/50 acetonitrile/water |
|
Sample Diluent: |
15/5/3 acetonitrile/ methanol/water |
|
Flow Rate: |
Reported in figures |
|
Gradient: |
Reported in figures |
|
Injection Volume: |
Reported in figures |
2.0 mg of polar peptide comprised of the following 20 residues: 4 basic, 11 polar and uncharged, 3 nonpolar, and 2 acidic, were dissolved in 1.15 mL of sample diluent, producing a concentration of 1.77 mg/mL peptide solution. The sample diluent was a mixture of 15/5/3 acetonitrile, methanol, and water. The crude peptide solution was vortexed and filtered through a 13 mm, 0.45 μm GHP syringe filter, part number WAT200516.
31 mg of polar peptide were dissolved in 2.3 mL of the sample diluent for a final concentration of 13.5 mg/mL. The sample mixture was vortexed and filtered.
The analysis and isolation of polar peptides is often challenging because of the difficulty in ensuring the retention of very hydrophilic sequences on a reversed-phase column. Hydrophilic Interaction Chromatography (HILIC), is an orthogonal chromatographic separation technique which separates hydrophilic compounds by their interaction with a polar stationary phase. Liquid-liquid partitioning, adsorption, ion exchange, and hydrogen bonding mechanisms all contribute to the retention of the sample. Analytes are eluted from the column by increasing the polarity of the mobile phase. The selectivity and retentivity of compounds on different stationary phases is dependent upon the specific properties of the column packing. As shown in Figure 1, the elution profile of the analytes is unique for each of the three HILIC stationary phases when the column dimensions and the chromatographic method are held constant. The BEH Amide column shows the most retention for the various types of compounds and a different selectivity compared to the other two columns. Better retention of similar compounds often improves the resolution between them.
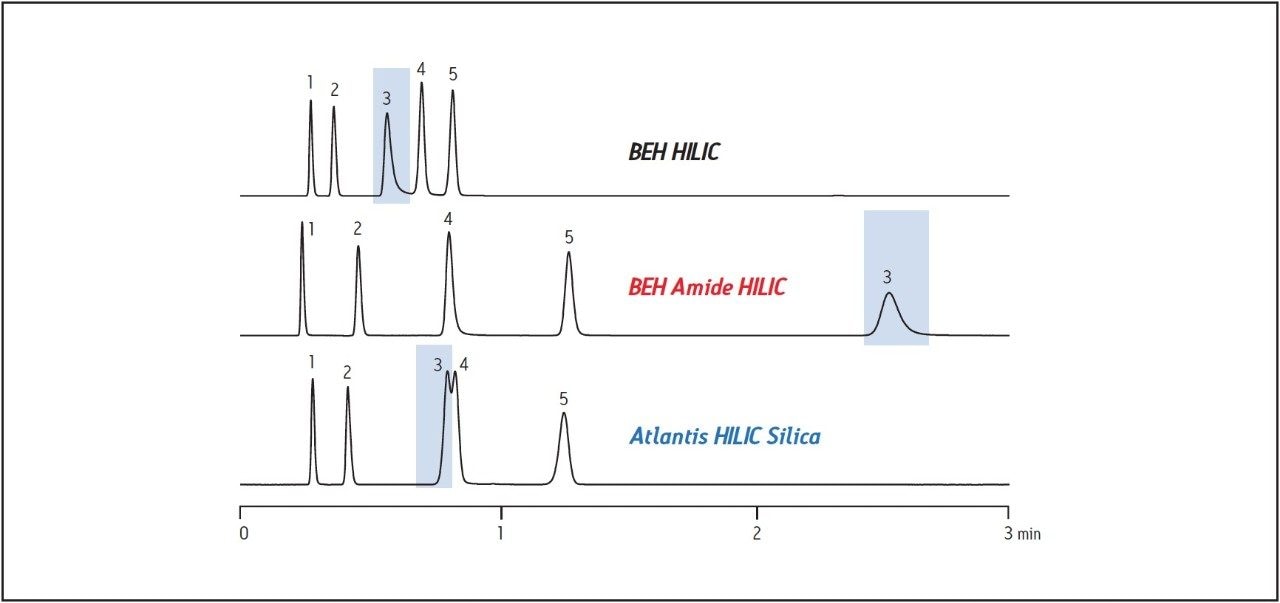
Although retention is crucial for effective separations, the nature of the target molecule must also be considered for a successful isolation of the compound. Deleted and failure sequences, adducts, and residual cleavage cocktail components contribute to the complexity of the crude sample mixture and complicate the isolation of the target peptide. The sample diluent also plays a role in retention, influencing solubility and peak shape. Traditional unbonded HILIC stationary phases usually require diluents and mobile phases with high organic concentration which limit the solubility of polar compounds at the high sample concentrations used in prep chromatography. Small amounts of water, even 10-20%, make the injection solvent incompatible with initial HILIC conditions on unbonded phases. Since the BEH Amide bonded phase tolerates mobile phases and injection solvents which are higher in aqueous content, polar peptides can be solubilized at concentrations amenable to preparative chromatography.
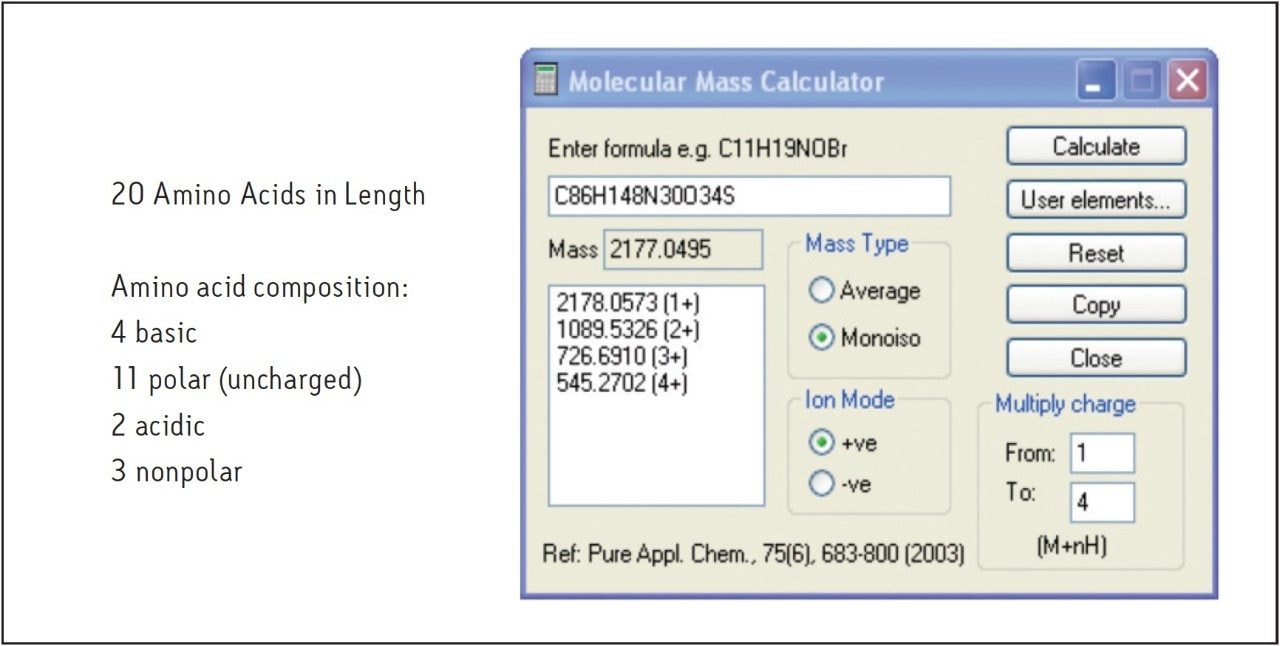
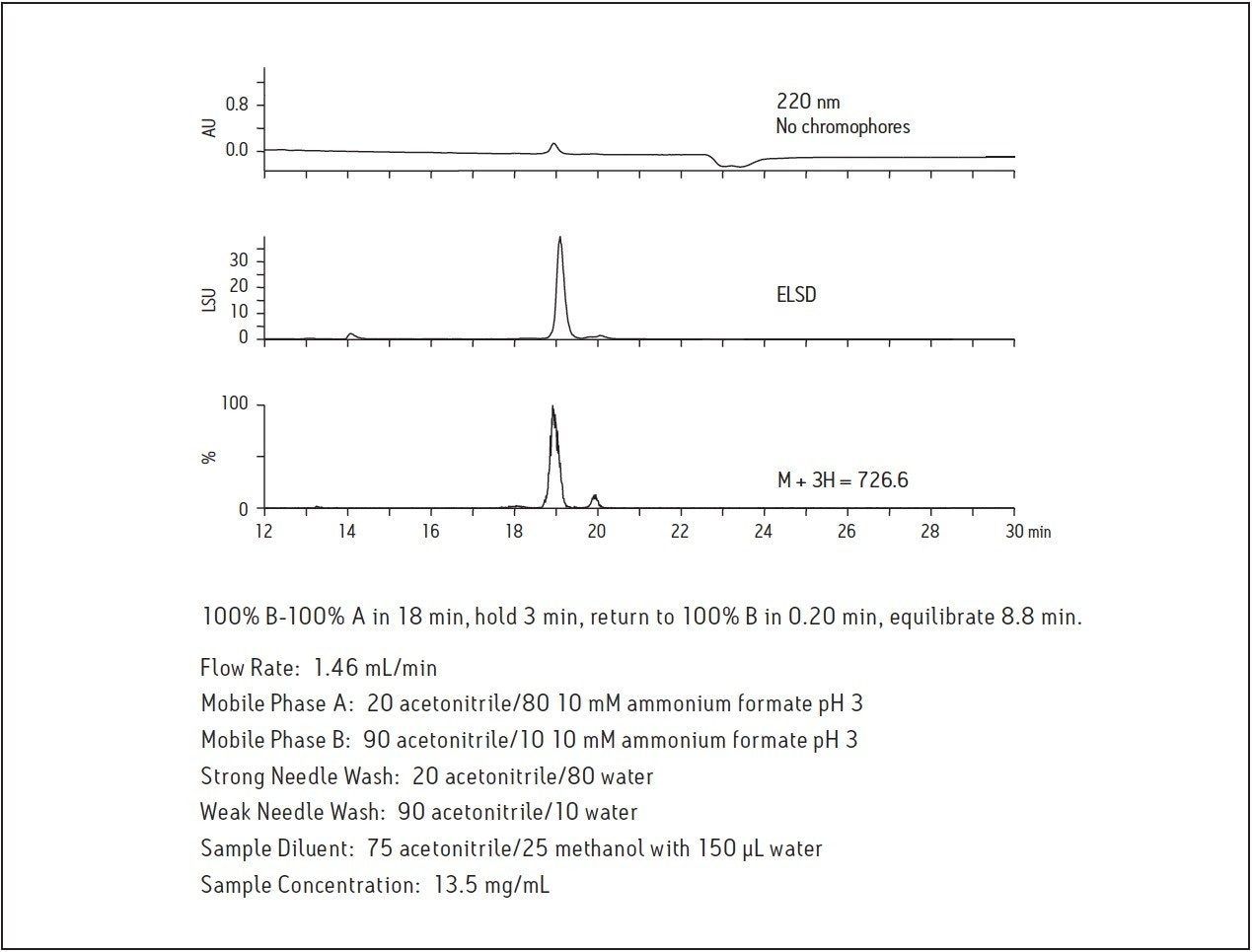
Because the amino acid sequence of the 20-mer in this study has no chromophores, peptide detection by UV is limited. Systems configured with alternate modes of detection identify target molecules with limited UV absorption or low ionization potential. Figure 2 shows the amino acid composition and the calculated monoisotopic mass and higher charge states for the peptide molecule used in this study. Since higher organic content mobile phases are typically used in HILIC, they are easily desolvated and provide an enhanced mass spectrometric response as well as faster fraction drying time. As shown in Figure 3, the peptide displays very little absorption at 220 nm due to the absence of chromophores in the amino acid sequence, but the ELSD and mass chromatograms have improved sensitivity, making isolation and analysis possible.
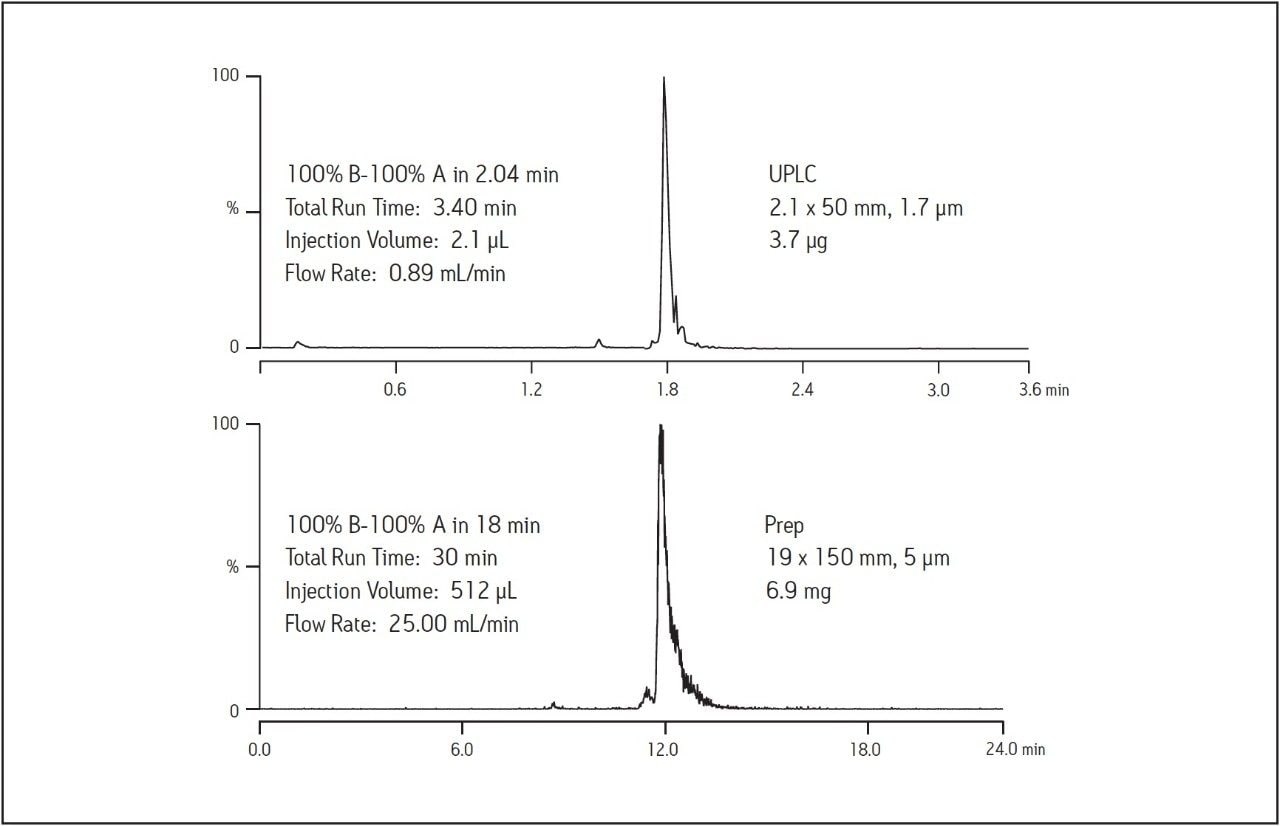
Scaling separations requires matching column chemistry as well as appropriately scaled gradients. As laboratories explore options for increasing throughput in the purification process, fast screening gradients using UPLC reduce the amount of time required for synthetic crude product analysis and, in some cases, fraction analysis. Since the BEH Amide column is available in sub-2-μm configurations, the synthetic crude peptide was analyzed using the ACQUITY UPLC. Maintaining the resolution between the UPLC and preparative scales requires the ratio of the length of the column to the diameter of the particle (or L/dp) remain constant. The UPLC, 2.1 x 50 mm, 1.7 μm column has an L/dp of about 29,400. The preparative, 19 x 150 mm, 5 μm column L/dp is 30,000, essentially equal to the L/dp ratio for the UPLC column. As expected, geometric scaling of the injection volume and chromatographic conditions produced a preparative chromatogram which is directly comparable to the sub-2-μm screening analysis done on the ACQUITY UPLC. Figure 4 illustrates the BEH Amide column scalability by comparing the chromatography using the fast screening gradient on UPLC and the larger scale chromatography used for the isolation.
720004283, April 2012