This application demonstrates the UPLC separation using a CORTECS UPLC HILIC Column to significantly improve the retention and resolution of diquat and paraquat, allowing for detection down to 500 ppt by UV detection alone.
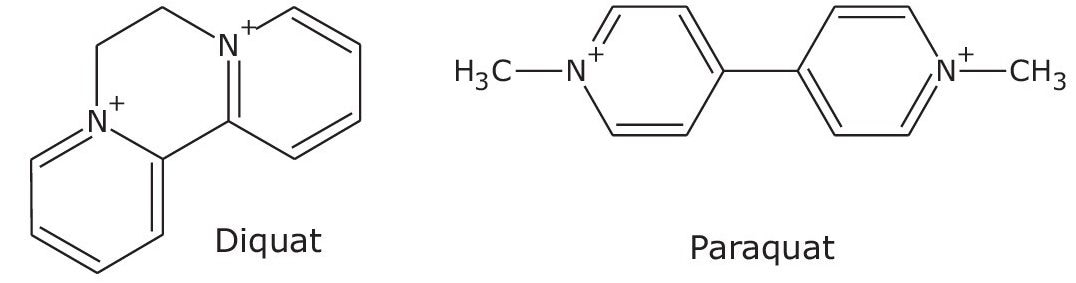
Diquat and paraquat are doubly charged quaternary ammonium herbicides (Figure 1). They have been and remain extensively used worldwide to control both crop and aquatic weeds. The United States Environmental Protection Agency (EPA) established the Maximum Contaminant Level (MCL) in drinking water at 20 µg/L (20 ppb). Paraquat is classified among the restricted use pesticides by the EPA. To ensure the quality of drinking water, the European Union (EU) Council set the minimum limit for individual pesticides at 0.1 µg/L (100 ppt).1 The use of paraquat was banned in the EU in 2007 following a legal case filed by the Swedish authorities. Both diquat and paraquat are too polar to be retained by reversed-phase liquid chromatography on C18 columns. Most published methods, including US EPA Method 549.2, must add ion-pairing reagents in the mobile phases, such as hexanesulfonic acid sodium salts, to achieve the necessary retention and sometimes resolution between the two “quat” analytes. Mass spectrometric (MS) detection may be necessary to meet the low quantitation requirement such as those mandated by the EU. The use of ion-pairing reagent will cause significant ion suppression for MS detection. As an alternate technique, Hydrophilic Interaction Liquid Chromatography (HILIC) has some advantages compared to the ion-pairing chromatography. MS ionization efficiency is improved because no ion-pairing reagents are added. Secondly, the extract that is typically in high percentage of organic solvent can be injected directly onto the column without dilution, with the aqueous mobile phase containing the ion-pairing reagent. This application demonstrates the UPLC separation using a CORTECS UPLC HILIC Column to significantly improve the retention and resolution of both analytes, allowing for detection down to 500 ppt by UV detection alone.
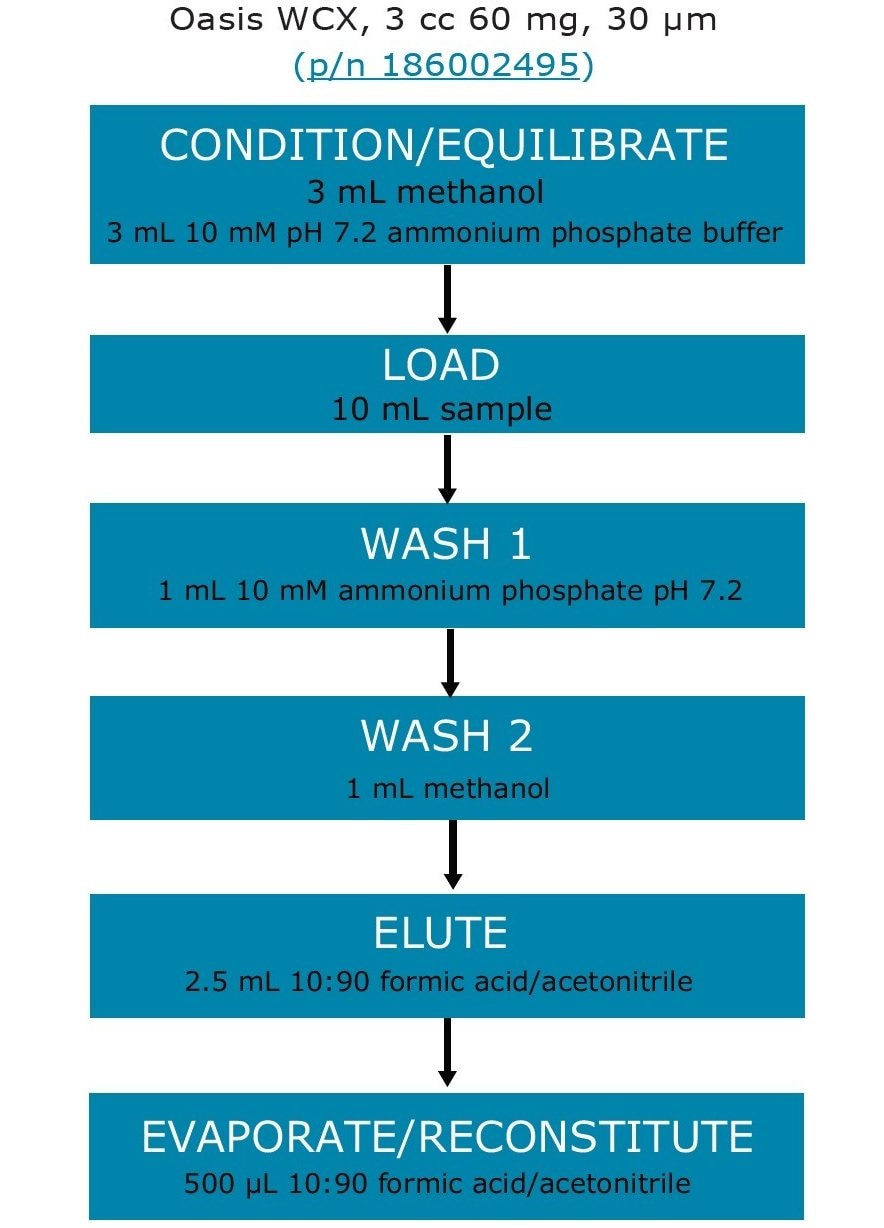
Tap water was first treated with sodium thiosulfate for dechlorination purposes. All samples were adjusted to approximately pH 7 by adding ammonium phosphate. Samples were then loaded to Oasis WCX cartridges. Sample enrichment was achieved by evaporation and concentration of the SPE eluents into smaller volume. The detailed SPE procedure is listed in Figure 2.
|
System: |
ACQUITY UPLC H-Class with photodiode array (PDA) detection |
|
Column: |
CORTECS UPLC HILIC 1.6 μm, 2.1 x 100 mm (p/n 186007106) |
|
Mobile phases (Isocratic): |
50:50 A/B |
|
Mobile phase A: |
200 mM ammonium formate buffer at pH 3.7 |
|
Mobile phase B: |
Acetonitrile |
|
Injection volume: |
20 μL |
|
Column temp.: |
30 °C |
|
Wash solvent: |
50:50 acetonitrile/water |
|
Purge solvent: |
50:50 acetonitrile/water |
|
Flow rate: |
0.5 mL/min |
|
PDA detection: |
Diquat UV at 308 nm, Paraquat UV at 257 nm |
|
Sample vials: |
Polypropylene autosampler vials (p/n 186002642) |
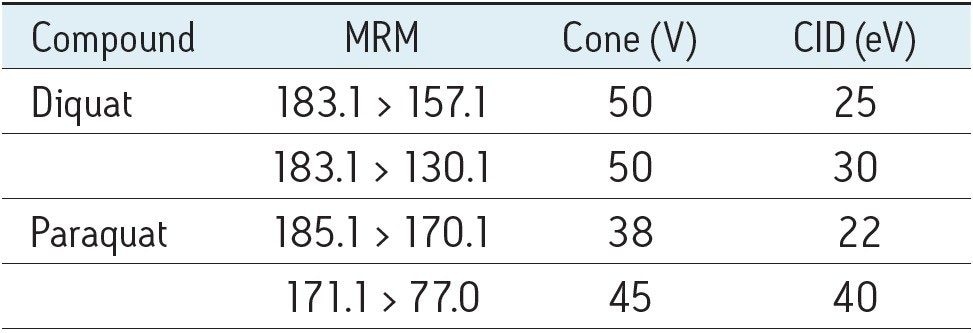
Table 1 summarizes the MRM transitions and LC-MS parameters used for this study.
|
Mass spectrometer: |
ACQUITY TQD |
|
Ionization mode: |
Positive electrospray |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
350 °C |
|
Desolvation gas flow: |
800 L/h |
|
Cone gas flow: |
30 L/h |
|
Collision gas flow: |
0.20 mL/min |
|
Data management: |
MassLynx Software |
Note: Polypropylene containers should be used for sample collection and for all sample preparation steps. Polypropylene autosampler vials (pn 186002642) are recommended for UPLC analysis.
1. Sample pre-treatment
Transfer a 10-mL sample to an appropriate polypropylene container (15-mL centrifuge tubes were used for this study). For chlorinated samples, add 50 µL of 20 mg/mL sodium thiosulfate and mix well. For all samples, adjust pH by the addition of 25 µL of 400 mM pH 7 phosphate buffer.
2. SPE enrichment and cleanup
Perform SPE enrichment and cleanup using Oasis WCX cartridges (see SPE details in Figure 2). To allow convenient loading of the 10-mL sample, attach a 30-cc polypropylene reservoir (p/n WAT011390) to each cartridge.
In a previous publication,2 the ACQUITY UPLC BEH HILIC Column was used for the analysis of diquat and paraquat in drinking water. The method is sensitive using MS detection with LOQ at 40 ng/L. However, UV-based detection cannot be used due to the lack of baseline resolution between diquat and paraquat (Figure 3A). When the same instrumental parameters are used for the CORTECS UPLC HILIC Column, there is an increase in both the retention and resolution of the two compounds (Figure 3B). As a result, the use of the CORTECS UPLC HILIC Column allows for detection using UV as well as MS. In order to optimize peak shape and analysis time, the final concentration of the ammonium formate buffer in mobile phase A was increased from 150 mM to 200 mM, and the mobile-phase composition was adjusted from 40:60 A/B to 50:50 A/B.
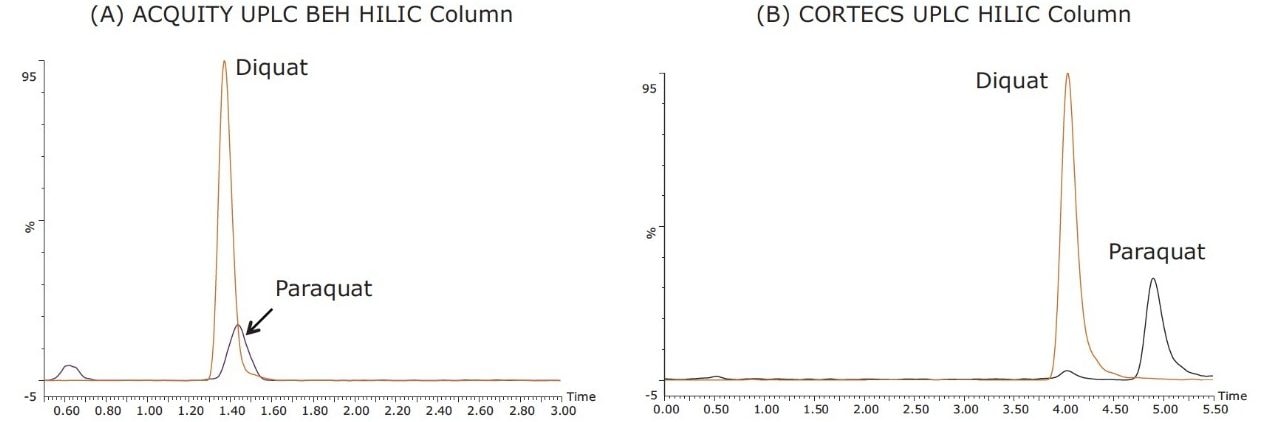
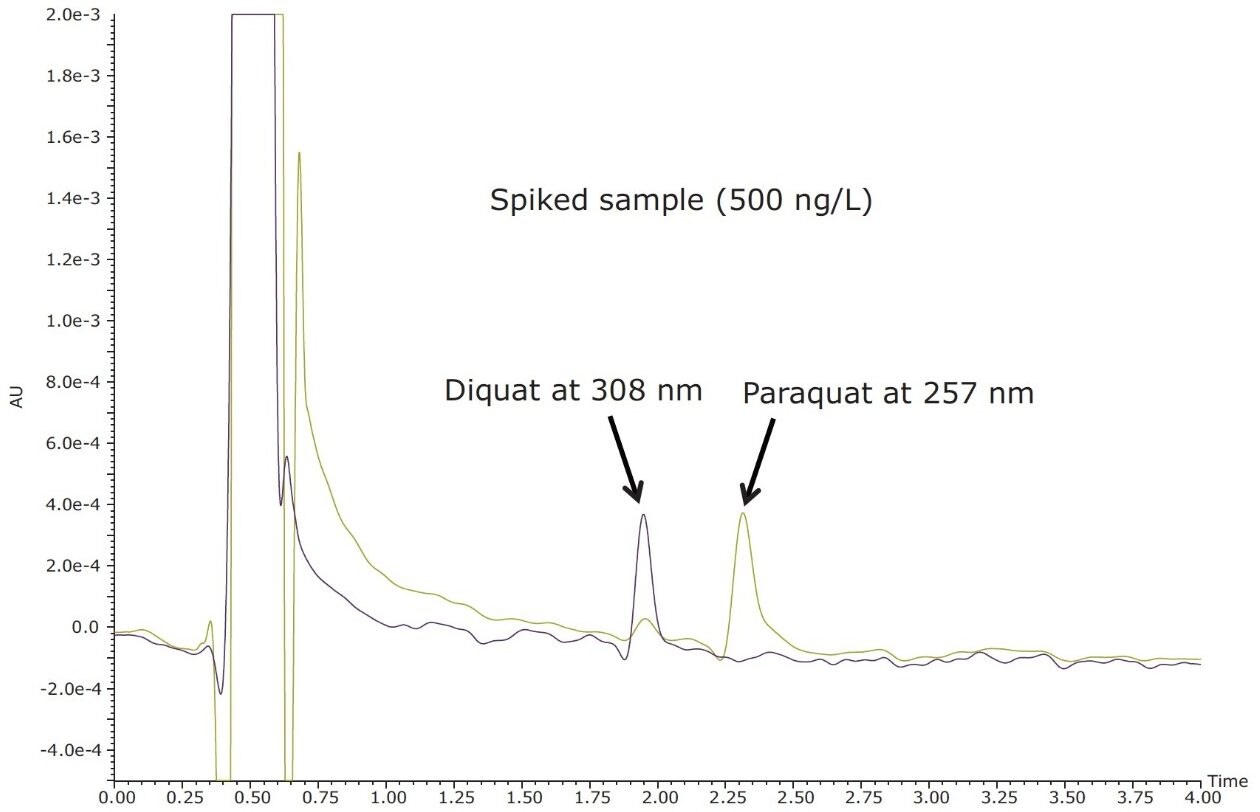
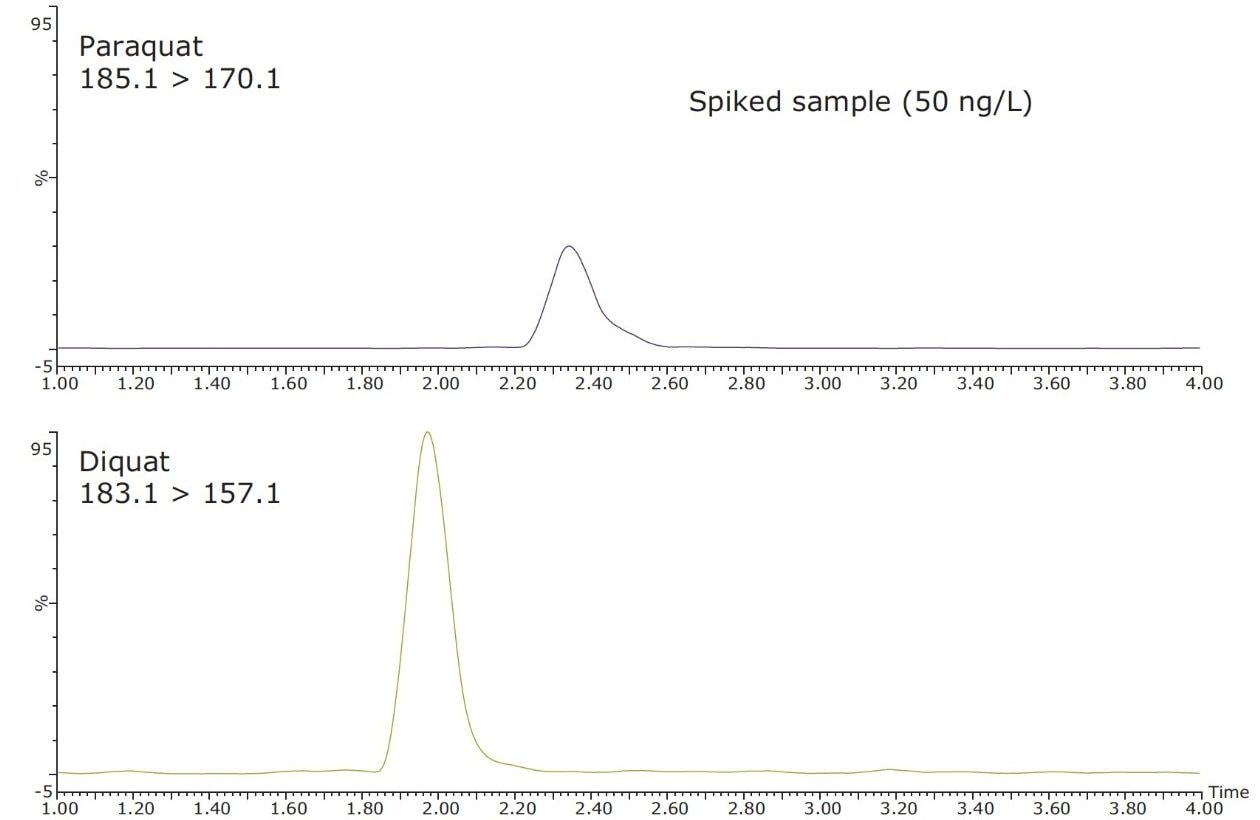
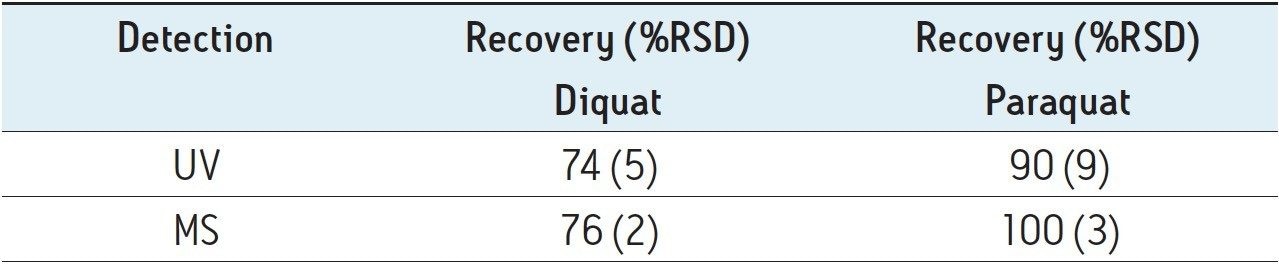
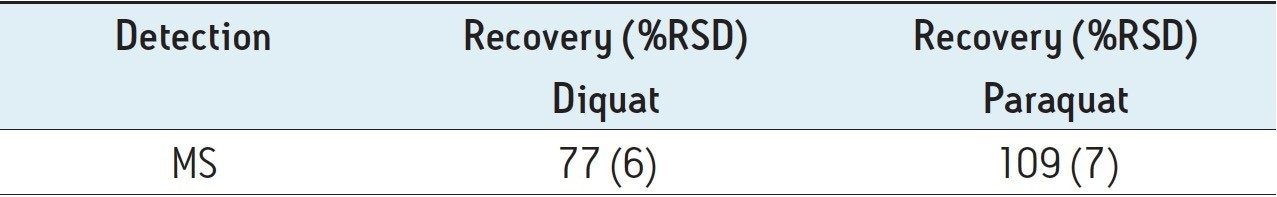
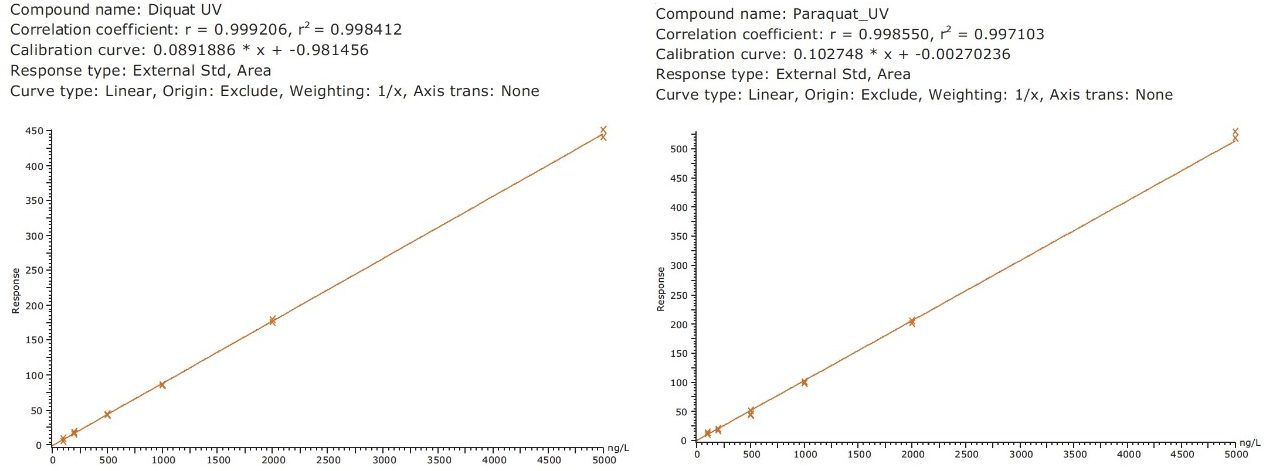
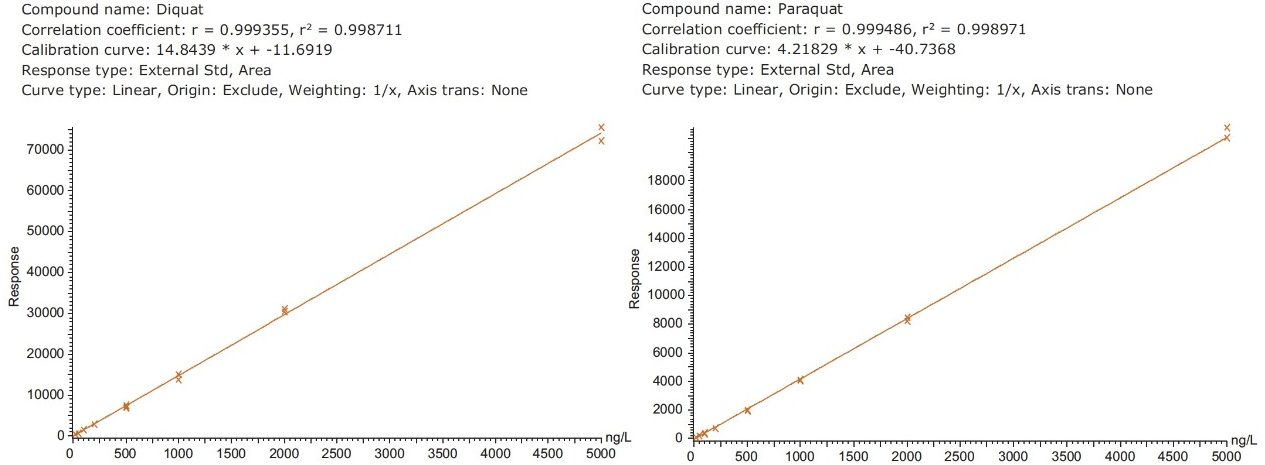
Figure 4 shows typical UPLC/UV chromatograms of a tap water sample spiked with diquat and paraquat at 500 ng/L prepared in tap water. UPLC coupled with the tandem MS technique is much more sensitive than the UV-based detection, thus allowing detection at a lower concentration of 50 ng/L. Figure 5 shows typical UPLC-MS/MS chromatograms of tap water sample spiked with diquat and paraquat at 50 ng/L prepared in tap water. Tables 2 and 3 show the recovery data obtained from replicate analyses of water samples spiked at 500 ng/L and 50 ng/L, respectively. Typical matrix-matched calibration curves were linear for both MS and UV detection. The standards used in calibration range from 25 to 2000 ng/L for MS detection, and from 100 to 5000 ng/L for UV detection. Calibration curves are presented in Figure 6 (UV) and Figure 7 (MS/MS).
The method performance is evaluated by Method Detection Limits (MDL) which is defined in the US EPA Method 549.2 by the following equation:
MDL = S t(n-1, 1-alpha = 0.99)
Where:
t(n-1, 1-alpha = 0.99) = student’s t value for the 99% confidence level with n-1 degrees of freedom
n = number of replicates (7)
S = standard deviation of replicate analysis
The results of MDL, calculated by using the recovery data from UV analysis of the tap water samples spiked at 500 ng/L, are summarized in Table 4. The method performance is equal to or better than the EPA Method 549.2.
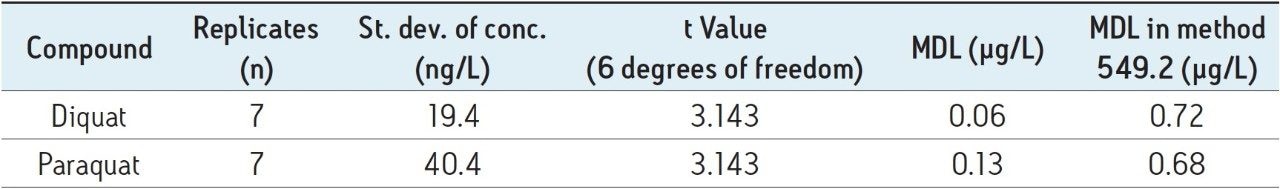
Due to the superior retention and resolving power of the CORTECS UPLC HILIC Column, diquat and paraquat peaks are baseline separated. This enables the use of the same chromatographic parameters for detection either by tandem MS or UV. By coupling UPLC with tandem MS, this method has sufficient sensitivity to satisfy the stringent sensitivity requirement at 0.1 µg/L for both compounds. The method using the UV detector alone has better performance than the EPA Method 549.2.
720004732, June 2013