
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates performance stability of CORTECS 2.7 μm Columns after repeated injections of precipitated plasma samples.
Robust performance results in extended column lifetimes.
Maintaining LC column performance is often a consideration when working with biological samples. In general, simple sample preparation techniques such as dilution or protein precipitation can result in reduced column lifetimes when compared to more selective sample cleanup procedures (e.g., solid-phase extraction [SPE]). CORTECS 2.7 μm Columns, made using optimally packed solid-core particles, are designed to be highly efficient, scalable, and robust. This includes the ability to maintain excellent chromatographic performance even after repeated exposure to minimally processed biological samples. This technical note details the ruggedness of a chromatographic separation of a panel of synthetic cannabinoid drugs and metabolites following repeated injections of precipitated plasma. These compounds represent a growing challenge for forensic and law enforcement laboratories. Due to the fact that new variants of these compounds are constantly being introduced, laboratories will often rely on relatively non-selective sample preparation techniques that ensure the efficient recovery of a wide variety metabolites and chemotypes. As a result, chromatographic columns need to be able to tolerate repeated injections of minimally processed samples, while maintaining assay performance.
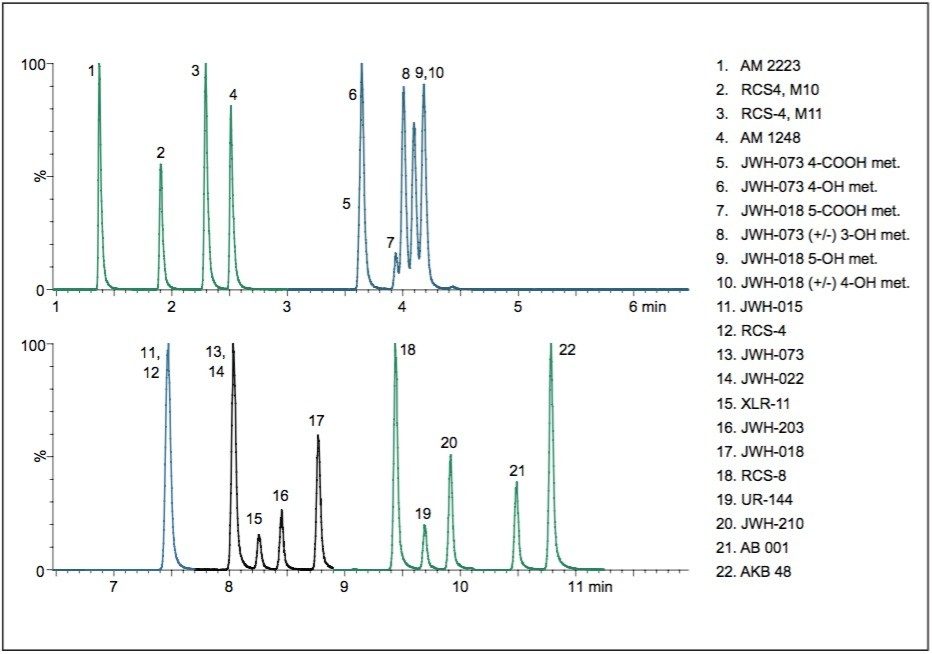
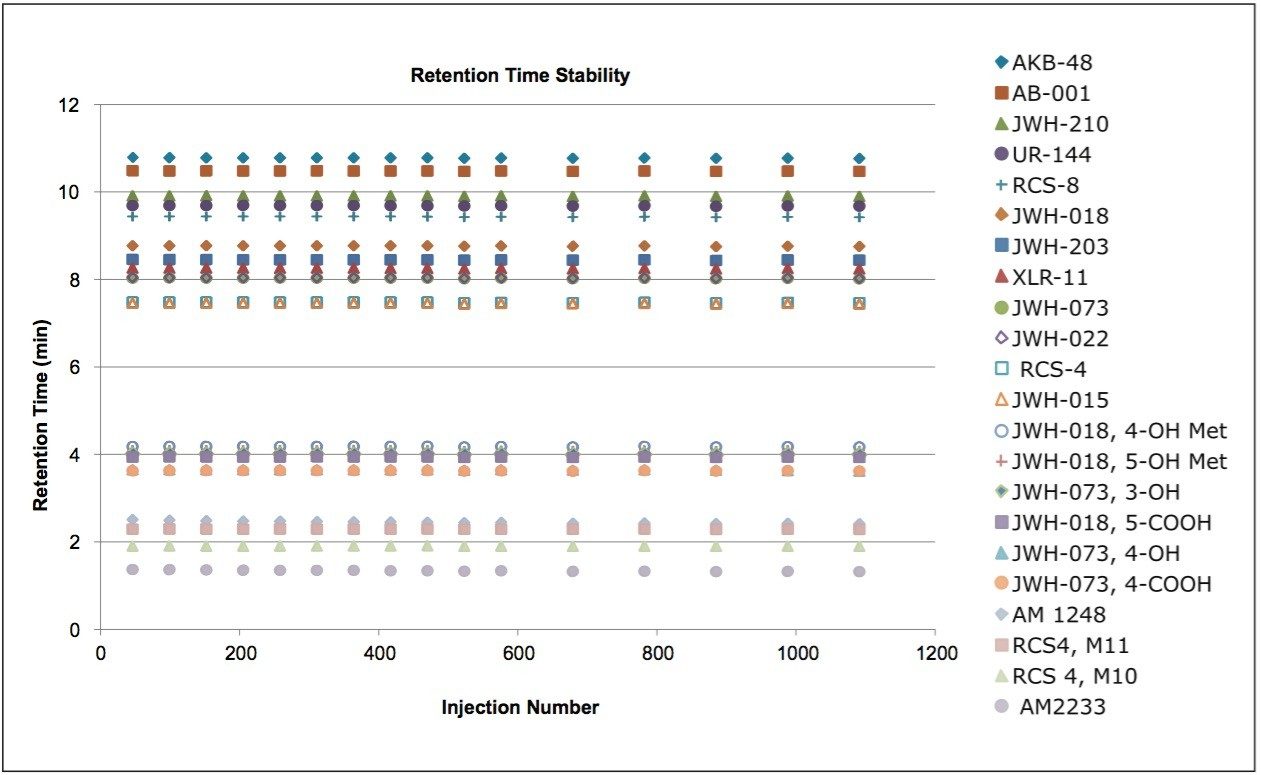
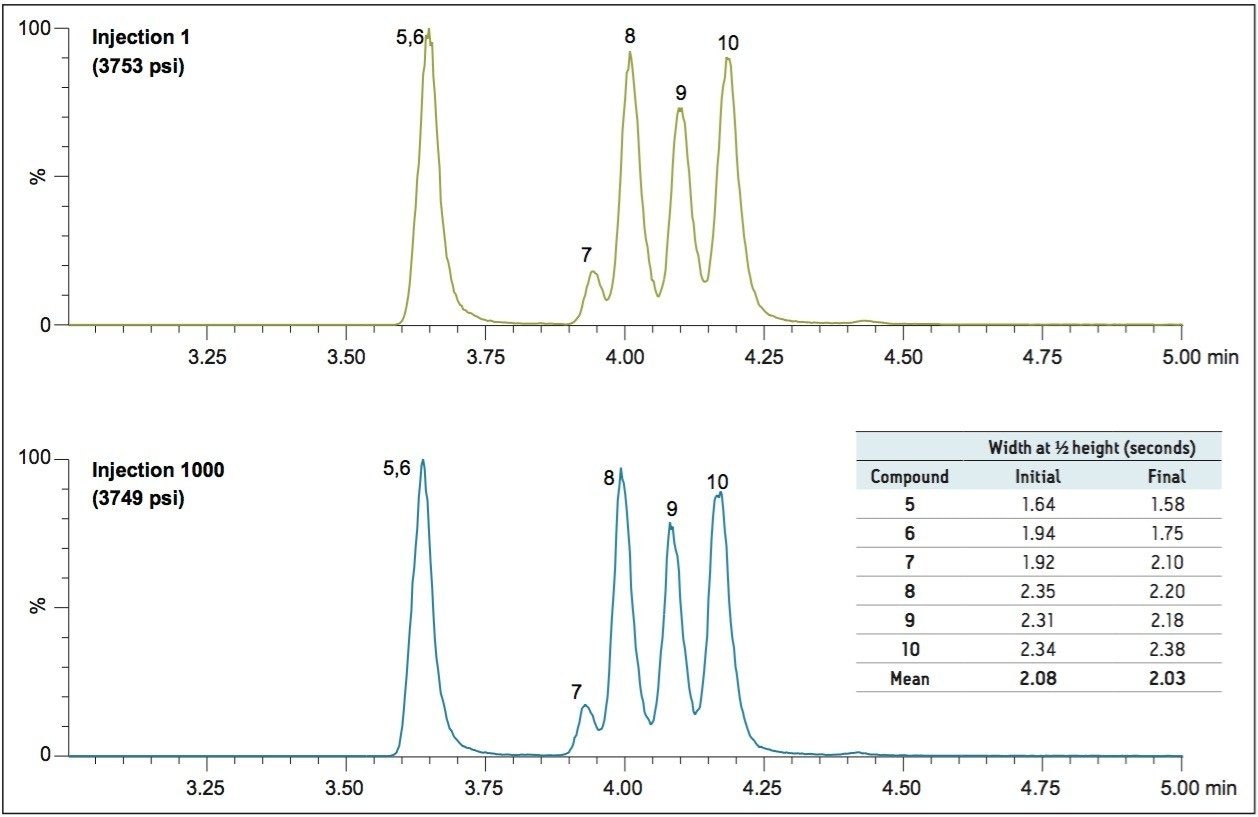
An LC-MS/MS method was developed for a panel of synthetic cannabinoid drugs and metabolites on a CORTECS 2.7 μm Column. This chromatography can be seen in Figure 1. The column was then challenged with 1,000 injections of plasma prepared using Ostro Sample Preparation Products. These plates are designed to remove precipitated proteins and phospholipids from biological matrices. The cannabinoid drug mix was then re-injected at intervals of 50–100 injections of prepared plasma. Figure 2 summarizes the retention time data for all 22 compounds over the entire experiment. Retention times for these analytes remained consistent, even after 1,000 injections of precipitated plasma. Further highlighting the chromatographic consistency, a section of the chromatogram containing two isobaric metabolites, compounds 9 and 10, is shown in Figure 3. The resolution between this critical pair of metabolites is unchanged, even after 1,000 injections of precipitated plasma. This figure also highlights the peak shape and retention time stability for the other 4 analytes. Over the course of the experiment, retention times for these analytes shifted by less than 1%. Mean peak widths at ½ height are shown in the adjacent table for the first 3 and final 3 injections. These data reveal no significant or systematic changes in peak widths. In addition, the maximum column backpressure was unchanged throughout the experiment. The initial maximum backpressure was 3,753 psi and the highest backpressure for the final injection was 3,749 psi, a change of only 0.1%. Thus, whether column performance is measured by retention time stability, maintenance of resolution, peak shape, or backpressure, the CORTECS Column demonstrates ruggedness that translates into extended column lifetimes.
The results presented here demonstrate the performance stability and ruggedness of CORTECS 2.7 μm Columns. Even after 1,000 injections of precipitated plasma, peak shape, retention times, and resolution between critical pairs of compounds is maintained. This ensures consistent chromatographic performance and long column lifetimes that translate into increased productivity for the analytical laboratory.
720005082, June 2014