
For research use only. Not for use in diagnostic procedures.
This application note demonstrates the extraction of cortisol, androstendione and 17-OHP from plasma samples using a novel SPE sorbent, Oasis PRiME HLB, in a μElution format for clinical research.The μElution format enabled the direct injection of extracts without evaporation or reconstitution.
Sample preparation is an important consideration for any bioanalytical LC-MS/MS method for clinical research. Waters has developed a novel sample preparation sorbent, Oasis PRiME HLB, which is designed to have some key advantages over traditional SPE sorbents. These include the ability to eliminate sorbent preconditioning and equilibration, allowing a more rapid workflow compared to traditional SPE products, and the ability to remove greater than 95% of phospholipids, resulting in a cleaner extracts and reducing the risk of short column lifetimes or MS source fouling.
This application note details the extraction and UPLC-MS/MS analysis of 17α-hydroxyprogesterone (17-OHP), androstenedione (Adione), and cortisol using Oasis PRiME HLB. Measurement of these compounds by immunoassay can be prone to cross reactivity with antibodies of chemically similar compounds. Immunoassays must also be done individually, requiring separate samples and analyses for each compound. LC-MS/MS offers greater discrimination and selectivity and the ability to multiplex methods, allowing the simultaneous determination of multiple compounds. The use of Oasis PRiME HLB resulted in consistent and highly reproducible recoveries of all compounds with minimal matrix effects. Phospholipids were almost completely eliminated compared to protein precipitation. Finally, the use of Waters patented μElution format allowed for the concentration of the sample on the SPE column, eliminating the need to evaporate and reconstitute the sample. This resulted in a method that was linear, accurate and precise for all analytes, with limits of quantification of 50 pg/mL for androstendione and 17-OHP.
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC HSS T3 Column, 100Å, 1.8 μm, 2.1 x 50 mm |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Mobile phase A (MPA): |
Water with 0.1% formic acid |
|
Mobile phase B (MPB): |
ACN with 0.1% formic acid |
|
Purge solution: |
25:25:50 ACN:MeOH:Water |
|
Wash solution: |
10% ACN |
|
Time(min.) |
Flow (mL/min.) |
%A |
%B |
|---|---|---|---|
|
0.0 |
0.6 |
70 |
30 |
|
1.0 |
0.6 |
50 |
50 |
|
2.0 |
0.6 |
45 |
55 |
|
2.5 |
0.6 |
5 |
95 |
|
3.5 |
0.6 |
5 |
95 |
|
3.6 |
0.6 |
70 |
30 |
|
4.5 |
0.6 |
70 |
30 |
Table 1. Mobile phase gradient. The compositions of MPA and MPB are listed in the Methods section.
|
MS System: |
Xevo TQ-S Mass Spectrometer |
|
Ionization mode: |
ESI Positive |
|
Capillary Voltage: |
1.0 kV |
|
Cone voltage: |
Optimized for each analyte |
|
Desolvation Gas: |
1000 L/hr |
|
Cone Gas: |
150 L/hr |
|
Desolvation Temp.: |
500 °C |
|
Source Temp.: |
150 °C |
|
Data were acquired and analyzed using MassLynx Software (V4.1; SCN 876). Quantification was performed using TargetLynx. |
All standards and stable isotope labelled internal standards were purchased from Cerilliant (Round Rock, TX). A combined stock standard (20 μg/mL cortisol; 1 μg/mL androstenedione and 17-OHP) was prepared in 25% methanol. A stock solution of 10 μg/mL cortisol-d4 and 0.5 μg/mL androstenedione-13C3 and 17-OHP-d8 was prepared in methanol. A working internal standard solution of 750 ng/mL cortisol d4 and 37.5 ng/mL androstenedione-13C3 and 17-OHP-d8 was prepared in 25% methanol. Individual calibrators and quality control standards were prepared daily in 25% methanol. 25 μL of each working calibrator or QC standard was added to 475 μL of double charcoal stripped human plasma (Golden West Biological, Temecula, CA) to make calibration curves and QC samples.
Samples were prepared as follows: 20 μL of the working internal standard solution was added to 150 μL of each calibrator or QC sample. All samples were precipitated with 300 μL of a solution of 4:1 MeOH:89 g/L ZnSO4. The samples were aspirated several times to ensure full precipitation and then centrifuged at 3220 rcf for 10 minutes. 300 μL of the resulting supernatant was then added to 900 μL of 4% H3PO4 and aspirated to fully mix the sample. The resulting pretreated sample was then directly applied to the Oasis PRiME HLB μElution Plate in 2 aliquots. All wells of the SPE plate were subsequently washed with 2 x 200 μL aliquots of 25% methanol. The samples were then eluted with 2 x 25 μL aliquots of 90:10 ACN:MeOH and diluted with 25 μL of water. 7.5 μL was injected onto the UPLC-MS/MS system. The sample extraction procedure is summarized in Figure 1.
Figure 2 shows the chromatography of the three steroids from an extracted calibrator. All compounds eluted within 2 minutes. The HSS T3 column offered a distinct advantage over other C18 columns. Even though these compounds are not polar, the enhanced retentivity of the T3 column eliminated solvent effects observed on other columns when samples were injected in high proportions of organic solvent.
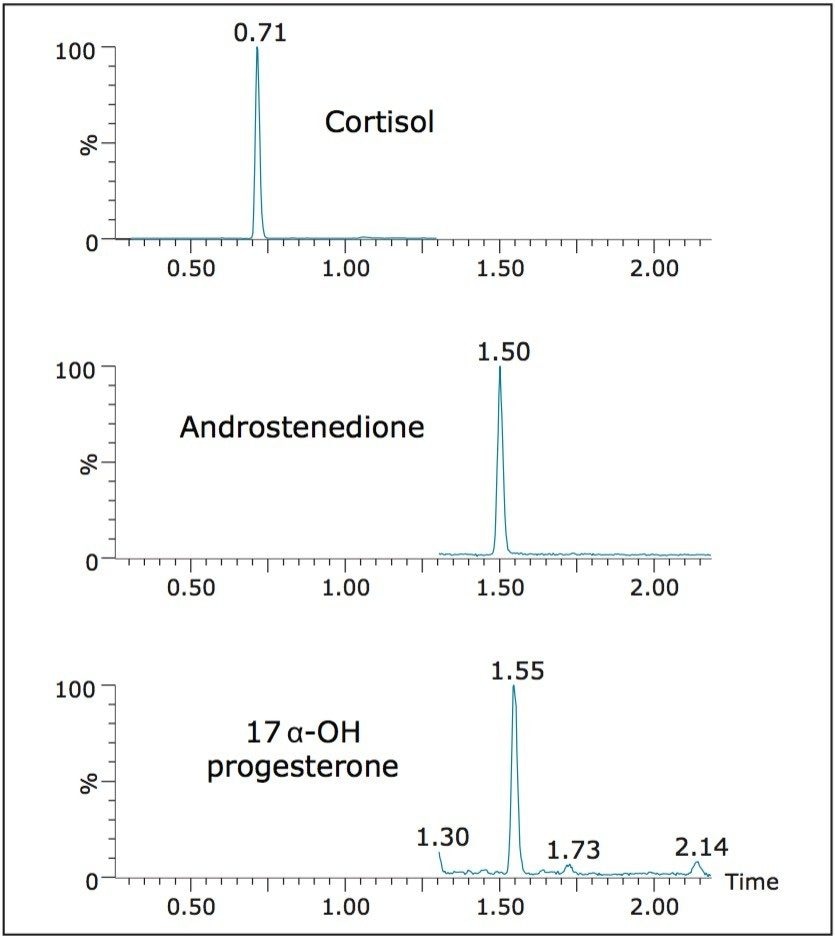
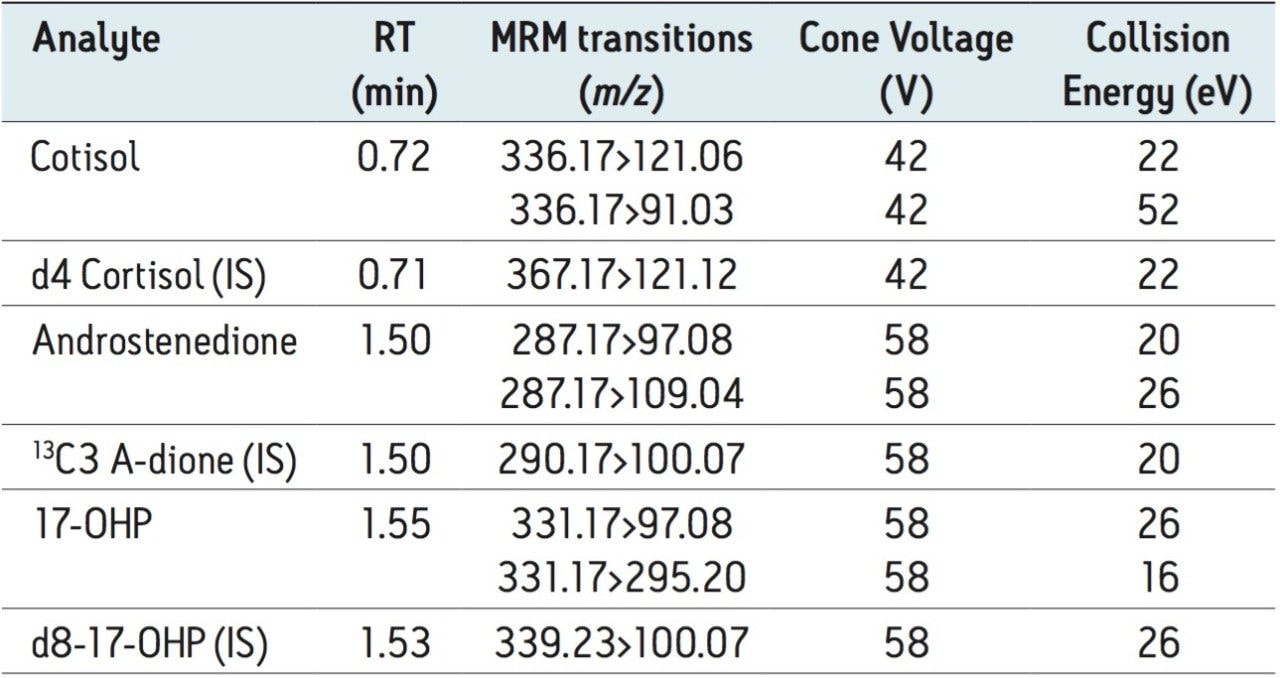
Table 2 lists the retention time and individualized MS parameters of the steroids and their stable isotope labelled internal standards, including MRM transitions, cone voltage, and collision energy. Two MRM transitions were used for each compound, a primary (listed first) and a confirmatory transition (listed second).
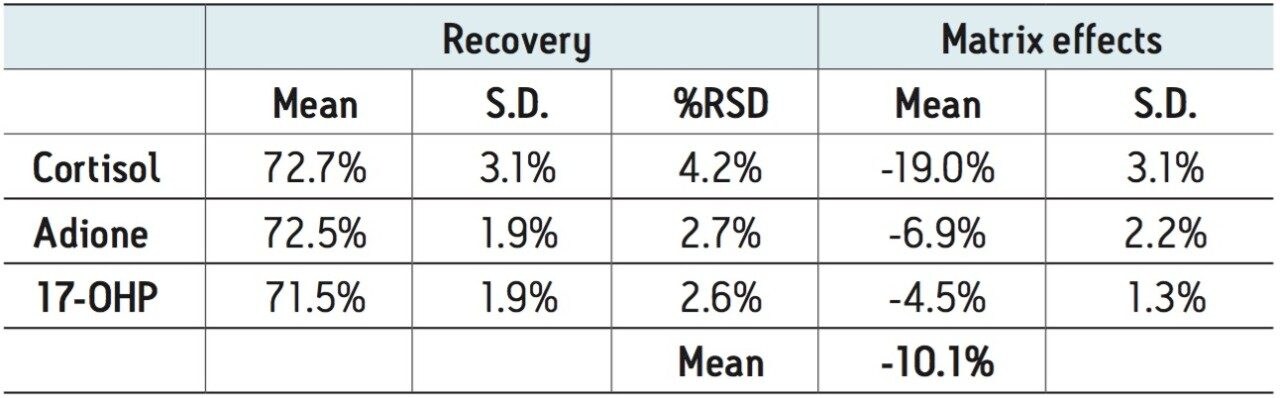
Extraction recovery was very consistent. As Figure 3 shows, recovery for all compounds was 72–73% with %RSDs under 5%, demonstrating the reproducibility of Oasis PRiME.
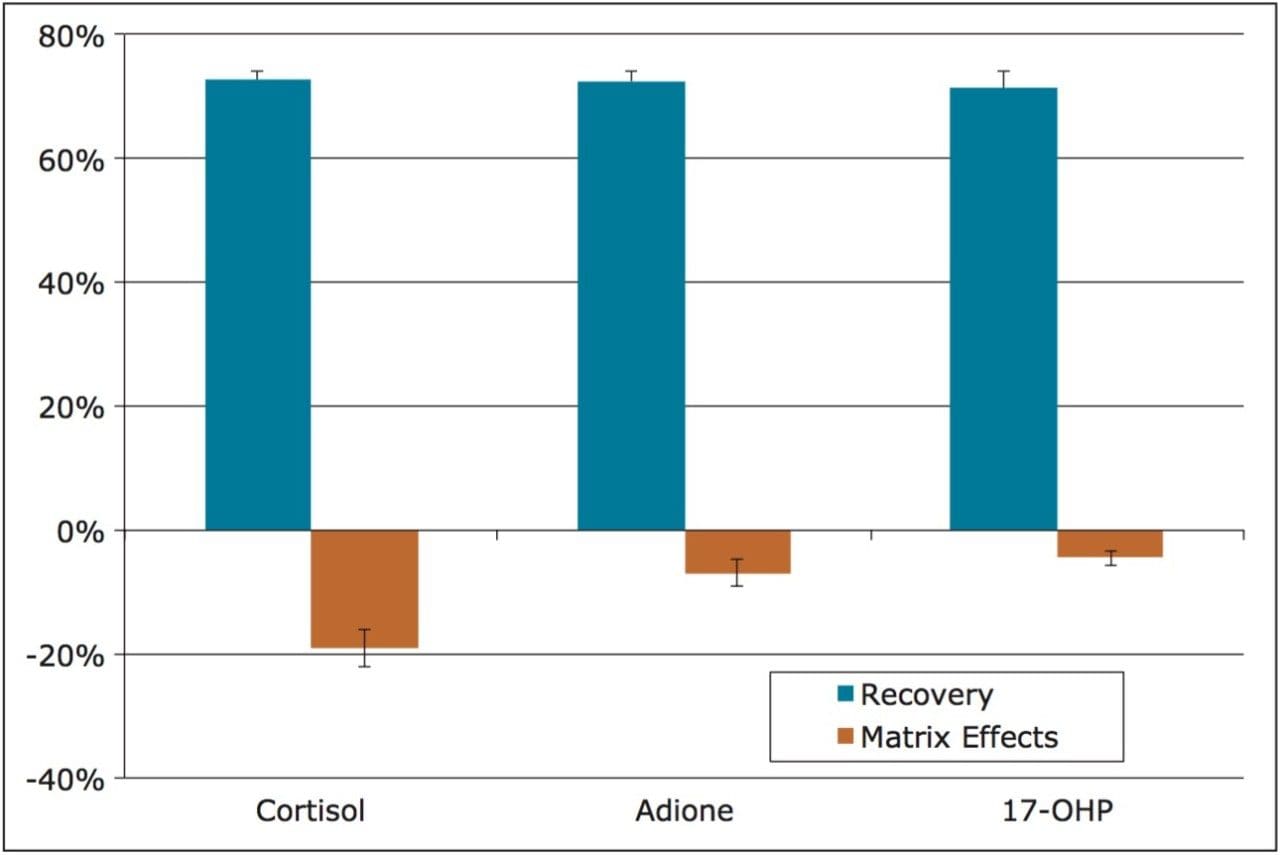
Matrix effects were low for all compounds. The matrix effect for cortisol was -19%, indicating minor ion suppression and was minimal for the other compounds. The average matrix effect for all compounds was -10.1%. Once again, the low standard deviations (3.1% or less) demonstrate the consistency of extraction and cleanup seen with Oasis PRiME. All recovery and matrix effect data are summarized in Table 3.
Calibration and quality control samples ranged prepared as previously described in the materials and method section. Calibration ranges were from 1–500 ng/mL for cortisol and from 0.05–25 ng/mL for the remaining compounds, mirroring the expected concentrations of these compounds in plasma. Quality control samples were prepared at low, medium, and high concentrations as appropriate for the calibration ranges.
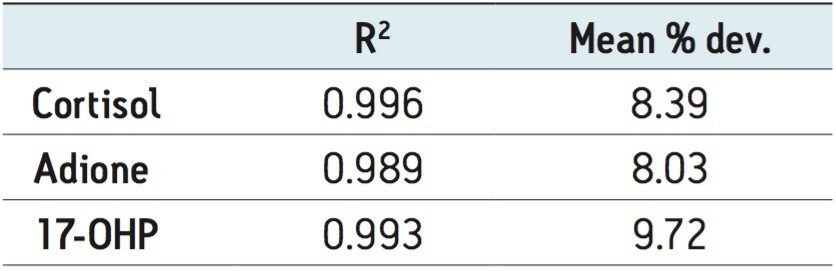
All compounds had linear responses over the entire calibration range with R2 values of 0.99 or greater with 1/x weighting. Table 4 summarizes the data from the calibration curves. Lower limits of quantification (LLOQ) were 1.0 ng/mL for cortisol and 0.05 ng/mL for androstenedione and 17-OHP. In each case, all FDA recommendations for accuracy, precision and analytical sensitivity were met for validated methods.1
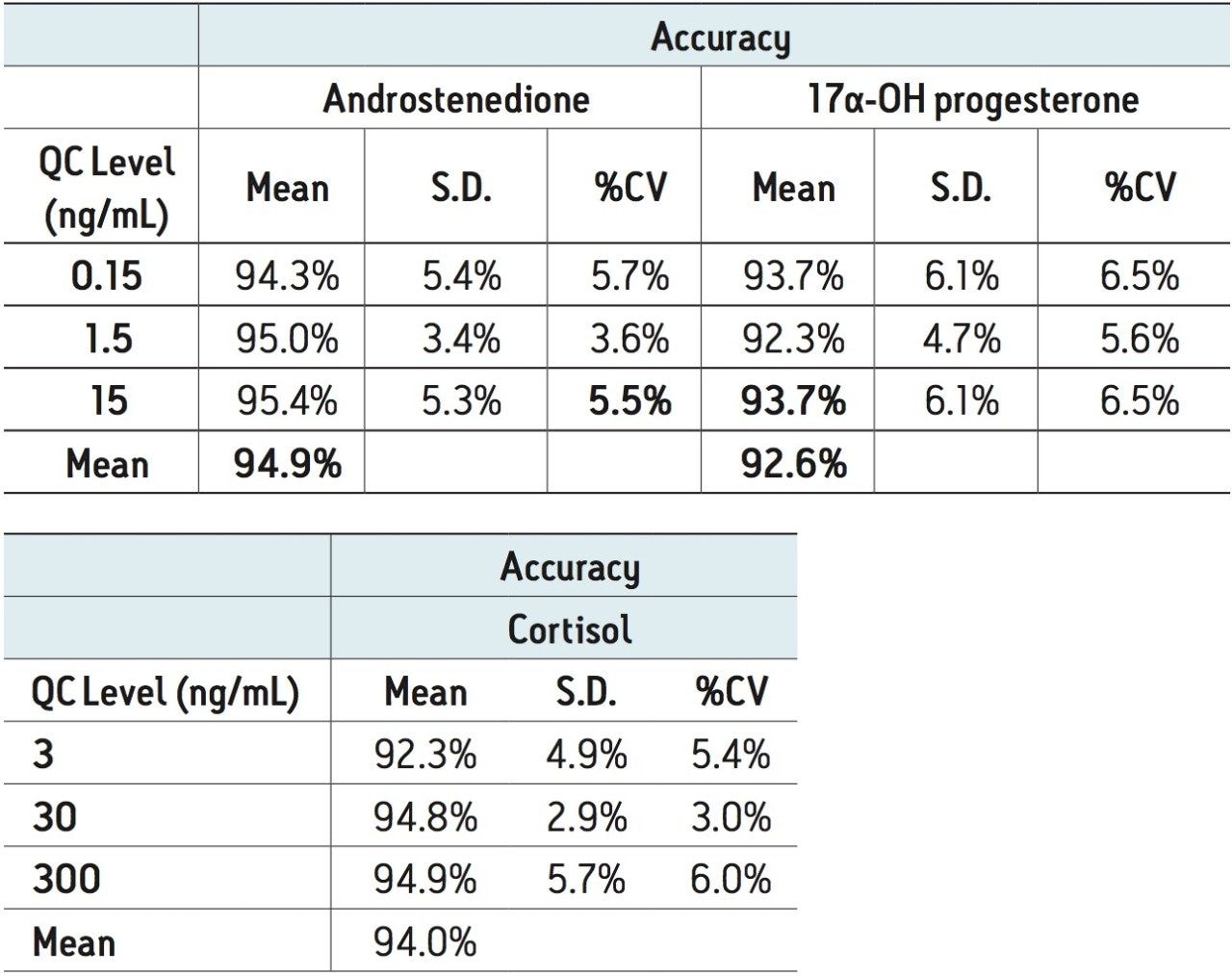
Quality control samples were accurate and precise, with all results within 10% of expected values and %CVs no greater than 7% (N=6). This data can be seen in Table 5. The excellent accuracy and precision demonstrate the consistency and robustness of this sorbent.
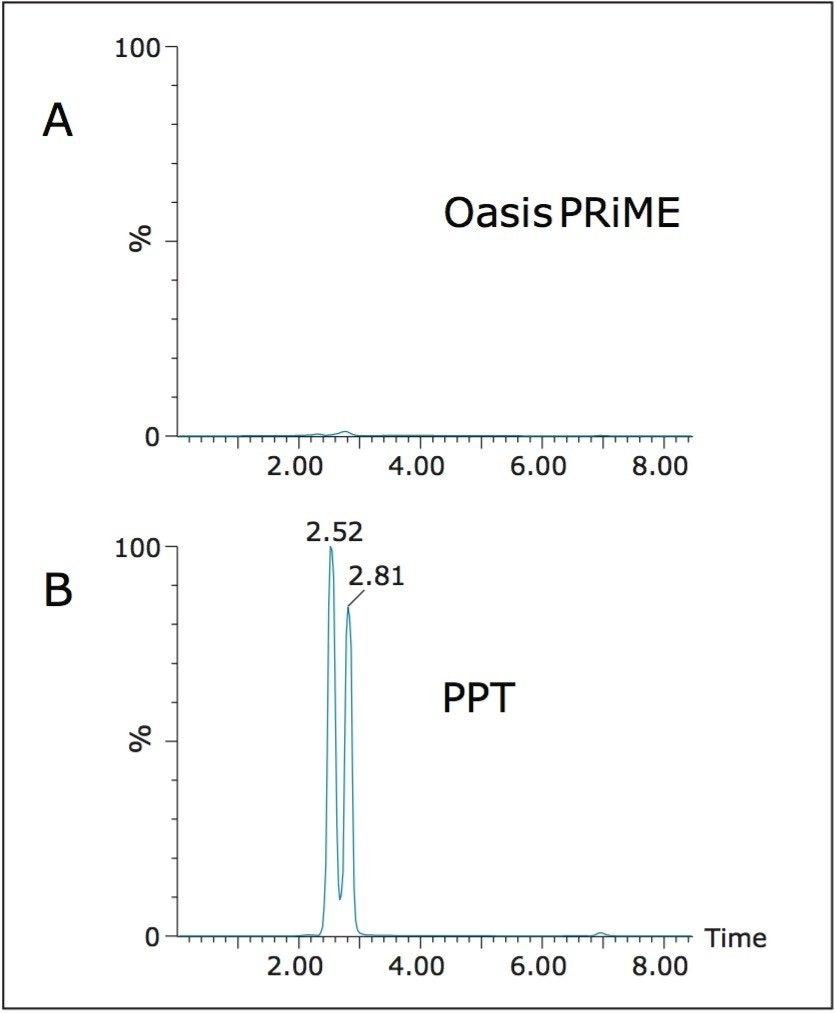
One of the key features of Oasis PRiME HLB over other reversed-phase SPE sorbents or liquid-liquid extraction is its enhanced ability to remove phospholipids, which can be major contributors to matrix effects,2,3 accumulate on analytical columns, and may contribute to fouling of MS sources, necessitating frequent cleaning. To assess the removal of phospholipids, we analyzed the extracts for several of the most common phospholipids and compared that data to samples that had been subject to protein precipitation only. Figure 4 compares chromatograms of identical plasma samples that were ether extracted as described above, or subject only to the protein precipitation step detailed. The total area of phospholipids in the Oasis PRiME HLB extracted samples was 3% that of precipitated samples. The true removal is likely even greater than 97% as there was no sample concentration for the precipitated samples. Taking the concentration factor into account (approximately 6X) would result in a removal of greater than 99%.
This application note details the extraction of cortisol, androstendione and 17-OHP from plasma samples using a novel SPE sorbent, Oasis PRiME HLB, in a μElution format for clinical research. The unique nature of this sorbent enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow. In addition, residual phospholipids were nearly eliminated compared to protein precipitation. The μElution format enabled the direct injection of extracts without evaporation or reconstitution.
Recoveries were very consistent for all compounds, and matrix effects were less than -20% for cortisol and under -10% for the other compounds. Linearity, accuracy, precision and analytical sensitivities were excellent for all compounds. All accuracies were within 8% of target concentrations and all %CVs were less than 7% for QC samples demonstrating the high reproducibility of this sorbet.
720005416, May 2015