This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to elucidate the complex N-glycans from erythropoietin using the GlycoWorks RapiFluor-MS N-Glycan Kit and hydrophilic interaction chromatography (HILIC).
To elucidate the complex N-glycans from erythropoietin using the GlycoWorks RapiFluor-MS N-Glycan Kit and hydrophilic interaction chromatography (HILIC).
Erythropoietin (EPO) is a highly glycosylated protein hormone that stimulates the production of red blood cells. EPO exhibits significant heterogeneity due to its multiple sites of glycosylation (three N-glycosylation sites at Asn 24, 38 and 83 and one O-glycosylation site at Ser 126) and the fact that each of these sites can bear various highly branched sialylated N-glycan structures.1-2 As a consequence of these post-translational modifications, an EPO will have an apparent SDS-PAGE molecular weight between 30 and 40 kDa, 40% of which corresponds to glycan content. Not surprisingly, the glycosylation of an EPO has been found to impact its therapeutic characteristics, namely its stability, efficacy and potency.1, 3-5 In vivo studies using glyco-engineered EPO have shown links between the safety and efficacy of a therapeutic EPO and several glycosylation associated critical quality attributes (CQA), perhaps with sialic acid content being the most important. Sialylated oligosaccharides have been shown to be associated with increased half-life in plasma as compared to desialylated forms which tend to be cleared within minutes.1, 3-5
Recombinant human EPO (rhEPO) expressed using Chinese hamster ovary (CHO) cells has been used efficiently in the treatment of anemia, since Epogen was approved by the FDA in 1989.6 As patents for EPO therapeutics approach expiration, the market for biosimilar rhEPO is expected to increase exponentially. Accordingly, there is a need for efficient and accurate methods that can be used for the characterization of EPO glycosylation.
N-glycans from rhEPO were released through fast enzymatic deglycosylation and rapidly labeled using a Waters GlycoWorks RapiFluor-MS N-Glycan Kit (p/n 176003635).7 The new RapiFluor-MS reagent has been designed to facilitate rapid labeling, improve fluorescence quantum yields and greatly enhance MS sensitivity.7 In this sample preparation, the complex N-glycans of EPO were first made accessible for enzymatic deglycosylation by the use of RapiGest SF, an anionic surfactant. Subsequently, its N-glycans were released in approximately 5 minutes using Rapid PNGase F and an elevated incubation temperature of 50 °C. The resulting deglycosylation mixture, containing free N-glycans (glycosylamines), was then subjected to a 5 minute labeling reaction with RapiFluor-MS. Labeled N-glycans were thereafter efficiently extracted from the reaction mixture using a GlycoWorks HILIC µElution plate (p/n 186002780) and GlycoWorks SPE Elution Buffer (p/n 186007992). This process of going from glycoprotein to extracted, labeled N-glycans was accomplished in 30 minutes. In addition, this sample preparation allowed for the immediate analysis of the RapiFluor-MS labeled N-glycans via a HILIC separation with a 2.1 mm x 150 mm, ACQUITY UPLC Glycan BEH Amide, 130Å, 1.7 µm Column (p/n 186004742) and an ACQUITY UPLC I-Class System. RapiFluor-MS N-glycan species eluting during these separations were serially detected by their fluorescence (FLR) and by positive ion-mode ESI-MS with a Xevo G2-S QTof Mass Spectrometer.
Figure 1 presents chromatograms from the HILIC-FLR-MS analysis of EPO N-glycans as labeled with RapiFluor-MS. Notably, both the fluorescence and base peak intensity (BPI) MS chromatograms showed high signal-to-noise such that the presence of different N-glycan species could be readily confirmed. The major N-glycans species in this profile were identified using the accurate mass information in combination with data from previous observations of EPO N-glycans.7 Previously, multidimensional chromatography strategies combining anion exchange chromatography and HILIC had been required to comprehensively characterize the N-glycans of EPO.7 In this work, we have been successful in identifying EPO N-glycans by employing a one dimensional HILIC separation along with online ESI-Q-Tof MS detection. This is an approach that is facilitated by the improved fluorescence and MS sensitivity afforded by RapiFluor-MS labeling.8 These new developments in N-glycan analysis aided in identifying tetra-antennary glycans with multiple sialic acids (three and four) as the most abundant species present on the analyzed rhEPO. The GlycoWorks RapiFluor-MS approach also helped in determining that tetra-antennary glycans with poly-N-acetyl lactosamine extensions were also present in relatively high abundance. Relative quantification from the fluorescence profile, in fact, showed that that the tetra-sialylated, tetra-antennary glycan species (FA4G4S4) represents approximately 20% of the total N-glycan pool, while tetra-antennary glycans carrying one (FA4G4Lac1S4) and two (FA4G4Lac2S4) lactosamine extensions constitute approximately 12 and 4.5% of the total N-glycans, respectively. More interestingly, GlycoWorks RapiFluor-MS approach has yielded identifications of tetra-antennary structures with three (FA4G4Lac3S4) and four (FA4G4Lac4S4) lactosamine extensions at levels of 0.75% and 0.25%, respectively. Although some previous studies on EPO have reported one and two N-acetyl lactosamine extensions, few studies have reported detailed information on species containing four or more lactosamine repeats.7 In this work, we have been able to successfully identify up to four repeats of poly-N-acetyl lactosamine using only a single dimension of separation and a gradient time of just 35 minutes (Figure 2). Moreover, it was possible to make confident identifications throughout this HILIC profile because of the enhanced fluorescence yields and the improvements in the ionization efficiencies of complex N-glycans that result from the use of the novel RapiFluor-MS labeling reagent.
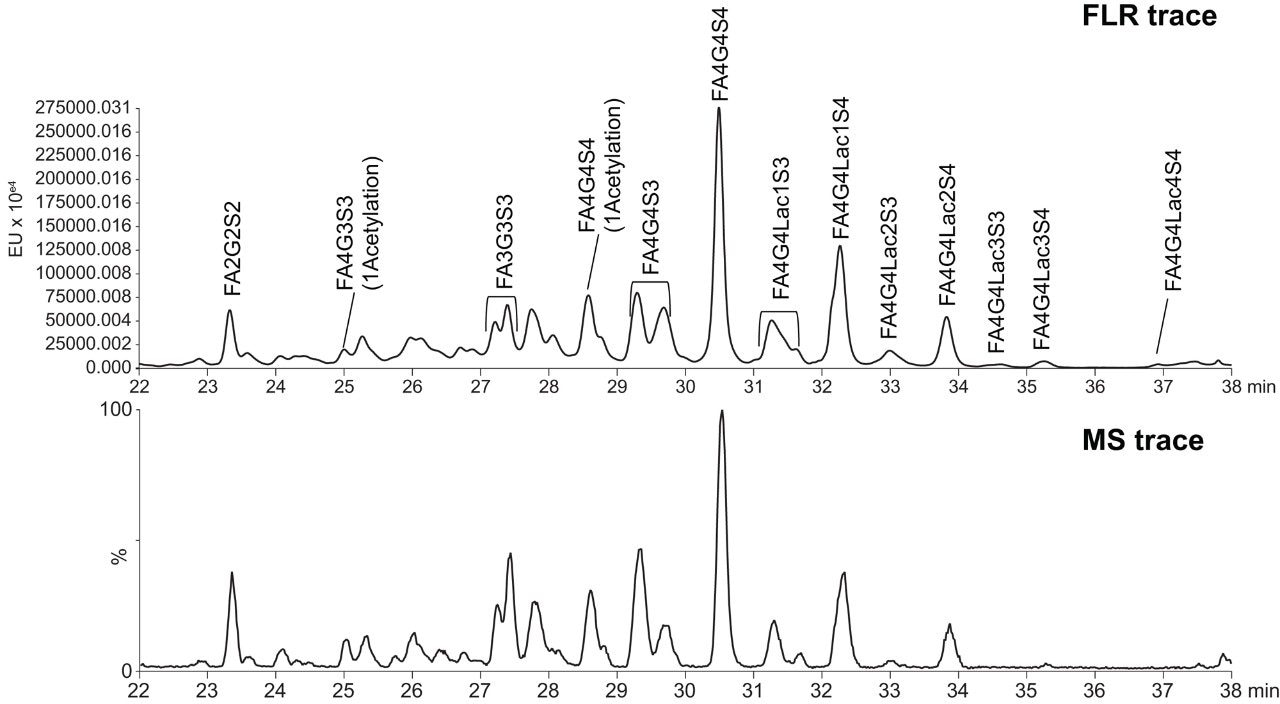
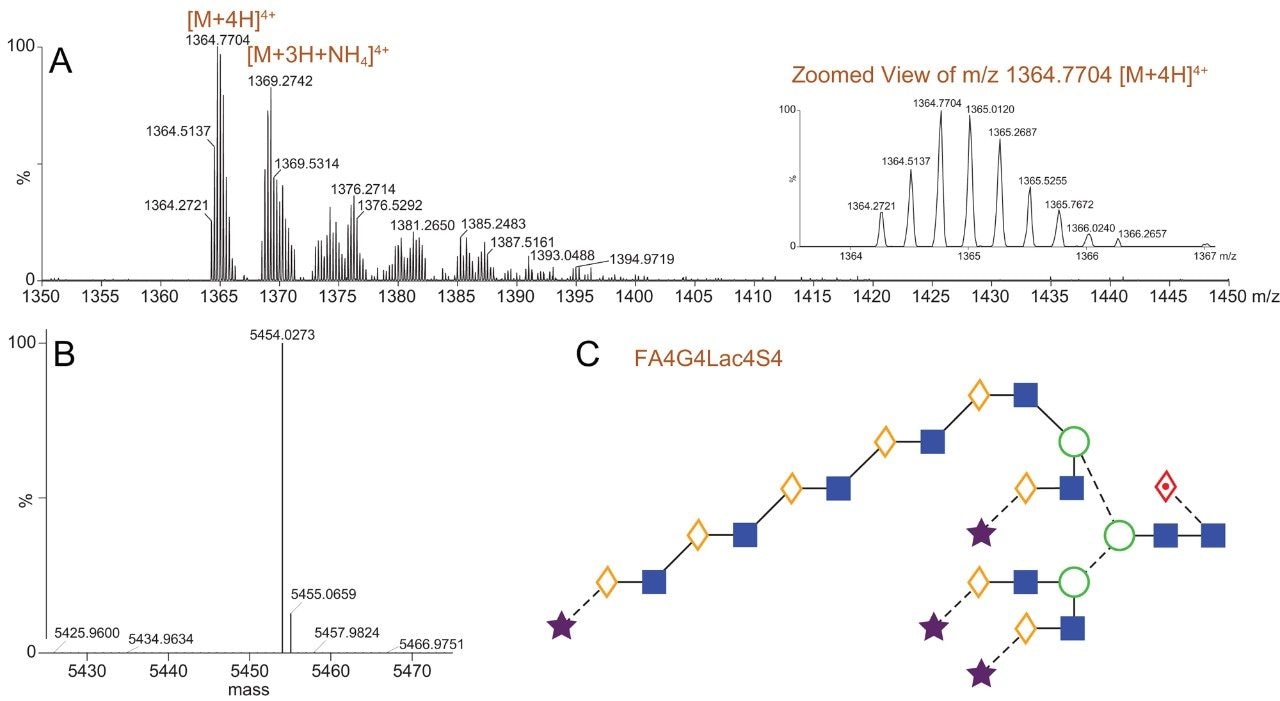
An approach combining the advantages of GlycoWorks RapiFluor-MS N-glycan sample preparation with the separation capabilities of UPLC HILIC has enabled us to perform a comprehensive analysis of the complex N-glycans present on a recombinant human erythropoietin (rhEPO). With the GlycoWorks RapiFluor-MS workflow, N-glycan samples were prepared in just 30 minutes. Most importantly, the samples were amenable to direct analysis by HILIC-ESI-QTof-MS analysis. RapiFluor-MS labeling not only reduced the burden of the sample preparation, but also enhanced the sensitivity of N-glycan detection, making it possible to obtain information-rich data and to elucidate the complicated N-glycan profile of an rhRPO. Because of these benefits, this new approach to N-glycan analysis could be used to hasten the development of EPO biosimilars.
720005444, July 2015