In this application note, we present a robust and quick UPLC method for analysis of methyl, ethyl, and isopropyl esters of benzenesulfonic acid.
The ACQUITY UPLC H-Class System, coupled with the ACQUITY UPLC PDA and ACQUITY QDa detectors, provides an excellent solution for monitoring potential genotoxic impurities with accurate confirmation and quantitation.
Overall, the ACQUITY QDa Detector is a robust and simple-to-use detector that can be added as an orthogonal detection technique to UV detection. It provides accurate and reliable results, making this technology ideal for routine testing of pharmaceutical products in the QC laboratory.
Genotoxic impurities (GTIs) are intermediates or reactants that can develop during the synthesis of a drug substance. In addition to process impurities, certain drugs may generate GTIs via degradation during formulation or storage.1 The genotoxic compounds have the potential to react with DNA, which could consequently cause a carcinogenic response and tumor development. It is therefore essential to identify the presence of these impurities early in the drug development process, and to have reliable and highly sensitive methods for their accurate determination in both drug substance and drug product.
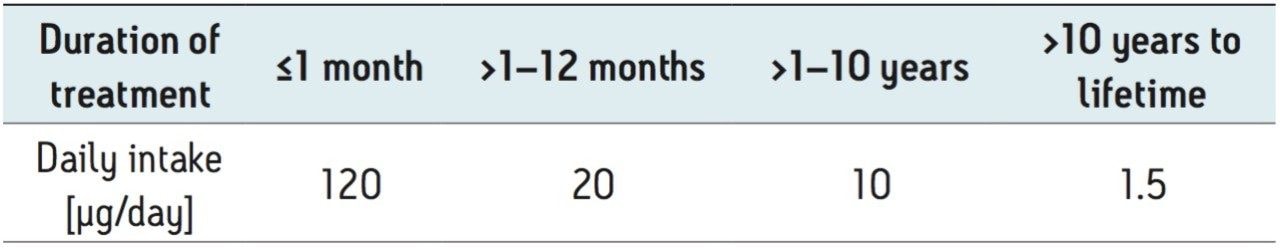
Regulatory agencies – including the European Medicine Evaluation Agency (EMEA), U.S. FDA, and International Conference on Harmonization (ICH M7) – have published guidelines on the allowable limits of genotoxic impurities in pharmaceutical ingredients to ensure the safety of pharmaceutical products.2-4 The guidelines require that any potential genotoxic impurities (PGIs) in a drug substance or drug product must be below the Threshold of Toxicological Concern (TTC) of 1.5 µg per day based upon the maximum daily dosage of the pharmaceutical compound over the lifetime of exposure. Daily intakes higher than 1.5 μg/day are acceptable for shorter exposure durations. The “staggered” TTC acceptable limits for less than a lifetime (LTL) to a lifetime exposure, as specified in the ICH M7 guidelines, are listed in Table 1.4
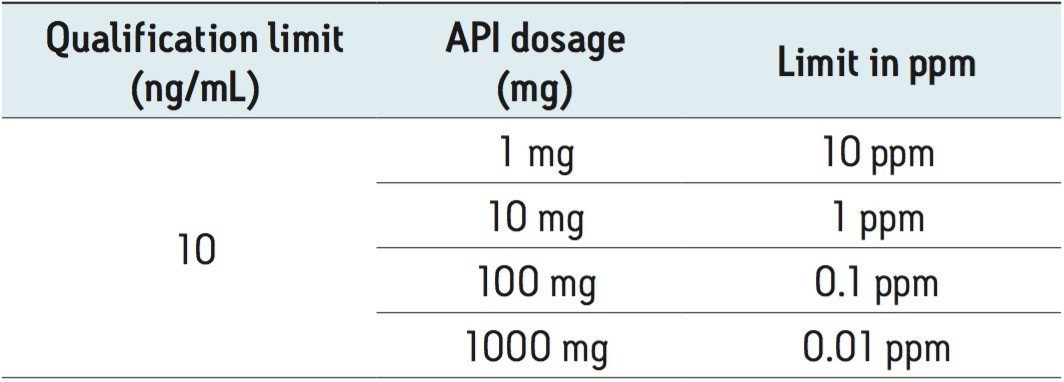
In the pharmaceutical industry, genotoxic impurities are typically reported relative to the API dosage in a pharmaceutical product. This means that a qualification limit of 10 ng/mL, for example, will correspond to 10 ppm for a product with 1 mg of API. Examples of qualification limits in ppm calculated for different API dosages are shown in Table 2.
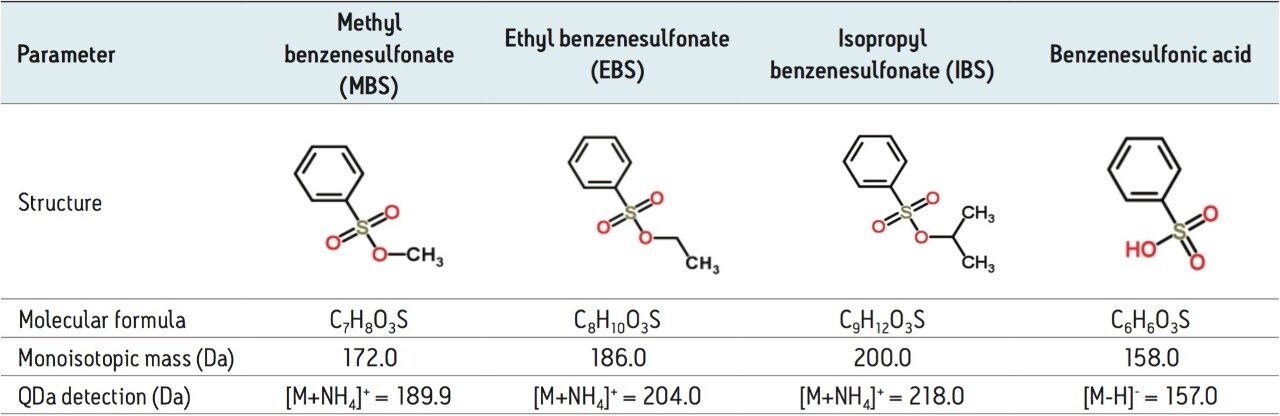
Sulfonic acids such as methanesulfonic acid (mesylate), benzenesulfonic acid (besylate), and p-toluenesulfonic acid (tosylate) are commonly used as counter ions with APIs to form salts. These sulfonic acids can interact with residual alcohols to generate alkyl sulfonate esters, which are considered PGIs.1 Current methods in literature for analysis of these alkyl sulfonate esters include liquid chromatography with MS detection and GC-MS.1 While effective, both techniques require a pre-column derivatization procedure to enhance detectability of the analytes, which is time-consuming and not ideal for routine testing. Mass detection coupled with UPLC enables direct and accurate analysis of alkyl sulfonate esters, eliminating the need for pre-column derivatization protocol. UPLC-MS provides the desired selectivity and sensitivity for quick analysis of low-level potential genotoxic impurities in pharmaceutical products.
In this application note, we present a robust and quick UPLC method for analysis of methyl, ethyl, and isopropyl esters of benzenesulfonic acid. The UPLC method uses UV and mass detection with an ACQUITY QDa Detector for fast and accurate monitoring of genotoxic impurities. We will demonstrate the linearity, sensitivity, and specificity of this method, which is achievable with both UV and mass detection. In addition, we will apply this method for the analysis of an amlodipine besylate drug substance. Overall, by employing mass detection we can enhance sensitivity and selectivity of the method, which is essential for analysis of low level impurities in pharmaceutical samples.
The materials used in this study include:
Separate stock solutions of methyl, ethyl, and isopropyl esters of benzenesulfonic acid were prepared in methanol at 1.0 mg/mL. An equal volume of each stock solution was transferred to one vial and diluted with standard diluent (20:80 methanol/5 mM ammonium acetate) to make a mixture solution containing 50 μg/mL of each analyte. The mixture solution was serially diluted with standard diluent (20:80 methanol/5 mM ammonium acetate) to make linearity standard solutions.
Linearity standards for UV detection were prepared at the following concentrations: 50, 100, 500, 1,000, 2,500, 5,000, 7,500, and 10,000 ng/mL. Linearity standards for MS detection included: 1.5, 7.5, 15, 25, 50, 75, 100, 250, and 500 ng/mL.
Amlodipine besylate API was dissolved in methanol at 2 mg/mL and then diluted with diluent (20:80 methanol/5 mM ammonium acetate) to 1 mg/mL.
|
LC system: |
ACQUITY UPLC H-Class |
|
Column: |
ACQUITY UPLC CSH C18, 1.7 μm, 2.1 mm x 50 mm |
|
Column temp.: |
40 °C |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
8.0 μL (optimized to 10 μL for methyl ester to improve sensitivity with MS detection) |
|
Solvent A: |
5 mM ammonium acetate in water (optimized to 1 mM of ammonium acetate for methyl ester to improve sensitivity with MS detection) |
|
Solvent B: |
Methanol |
|
Separation: |
Gradient |
|
Purge wash: |
50:50 water/methanol |
|
Sample wash: |
50:50 water/methanol |
|
Seal wash: |
90:10 water/acetonitrile |
|
UV detector: |
ACQUITY UPLC PDA, 200–400 nm, derived at 220 nm |
|
Step |
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
1 |
Initial |
90 |
10 |
Initial |
|
2 |
3.5 |
10 |
90 |
6 |
|
3 |
4.0 |
10 |
90 |
6 |
|
4 |
4.5 |
90 |
10 |
6 |
|
5 |
6.5 |
90 |
10 |
6 |
|
Mass detector: |
ACQUITY QDa (Performance option) |
|
Ionization mode: |
ESI+, ESI |
|
MS acquisition time: |
0–3.5 minutes |
|
MS acquisition range |
100–250 Da |
|
SIR(+): |
190.0, 204.0, and 218.0 Da |
|
Sampling rate: |
10 pts/sec |
|
Capillary voltage: |
positive 1.4 kV, negative 0.8 kV |
|
Cone voltage: |
6 V |
|
Probe temp.: |
300 °C |
|
Data: |
Centroid |
|
Data management |
Empower 3 CDS Software |
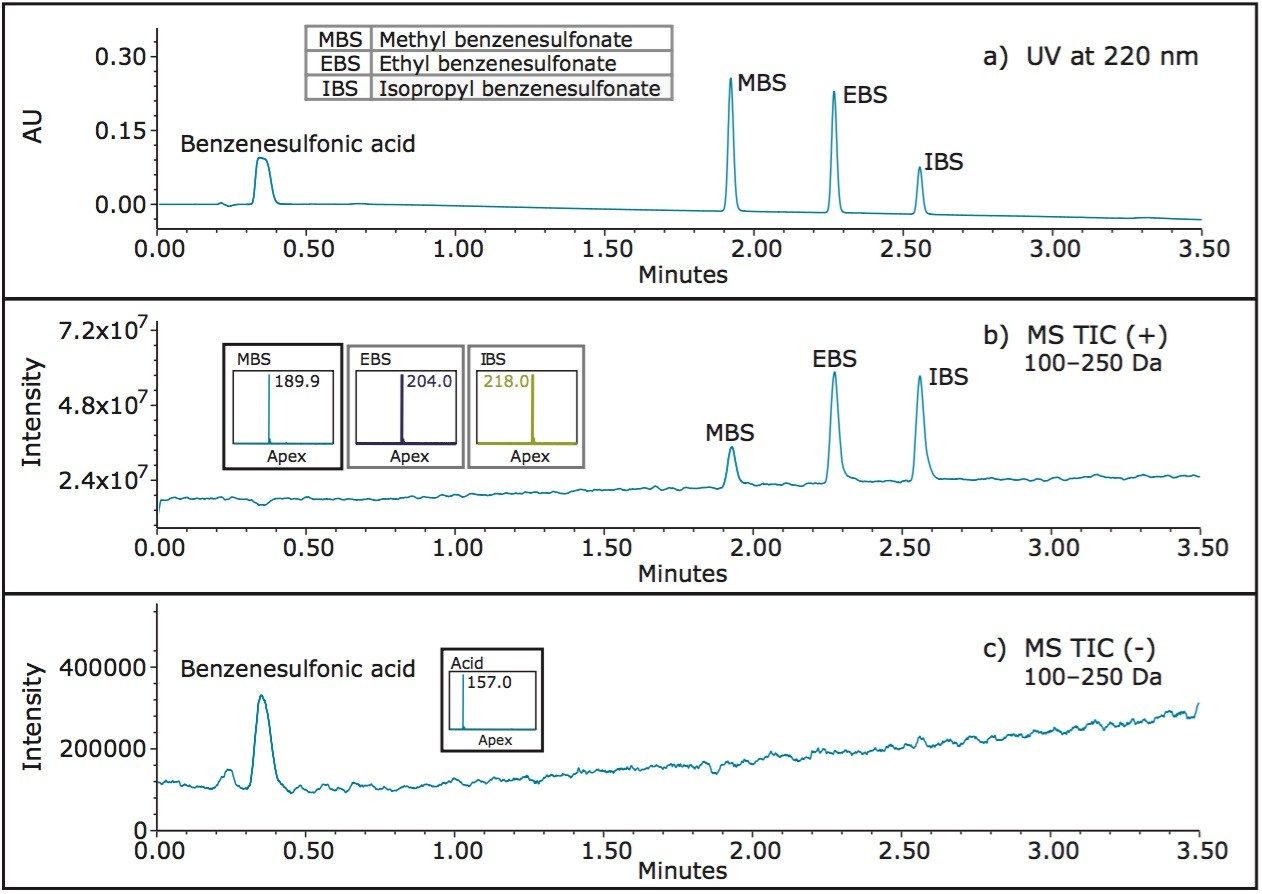
The chemical structures of methyl, ethyl, and isopropyl esters of benzenesulfonic acid are shown in Table 3. The UV and MS chromatographic data for the separation of these compounds is displayed in Figure 1. The UV trace (Figure 1a) shows that benzenesulfonic acid elutes before methyl, ethyl, and isopropyl esters. The ACQUITY QDa MS data collected across the mass range (100–250 Da, Figure 1b and 1c) is known as the total ion chromatogram (TIC), and shows that alkyl esters are detectable in ESI+ mode, whereas benzenesulfonic acid is detectable in ESI- mode. The mass spectral data indicates that the esters form ammonium adducts, [M+NH4].+
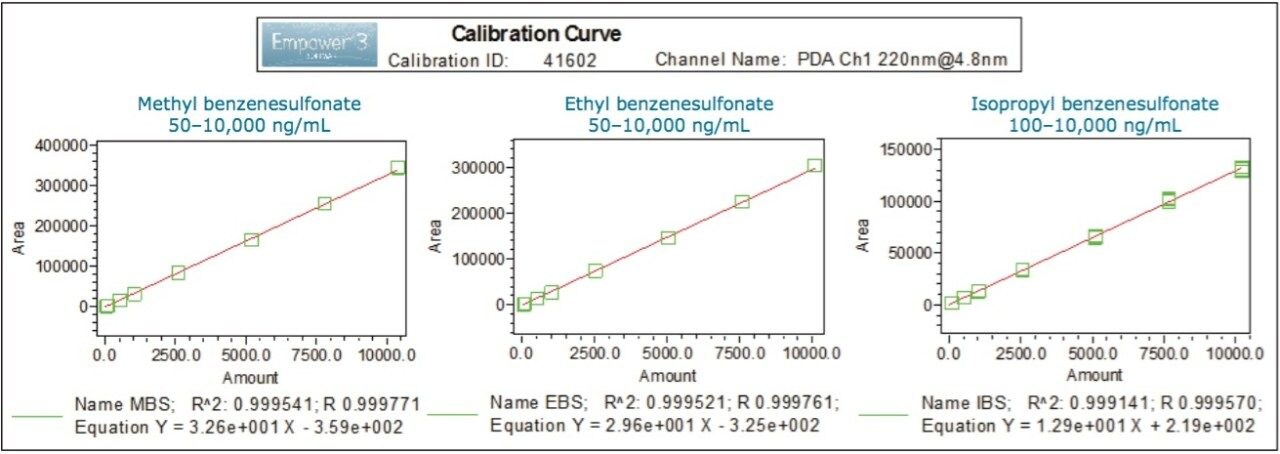
The UV data exhibited an acceptable linearity for methyl, ethyl, and isopropyl esters of benzenesulfonic acid over seven concentration levels, ranging from 50 to 10,000 ng/mL, with the correlation coefficients (R2) of ≥ 0.9991 (Figure 2) using linear fitting.
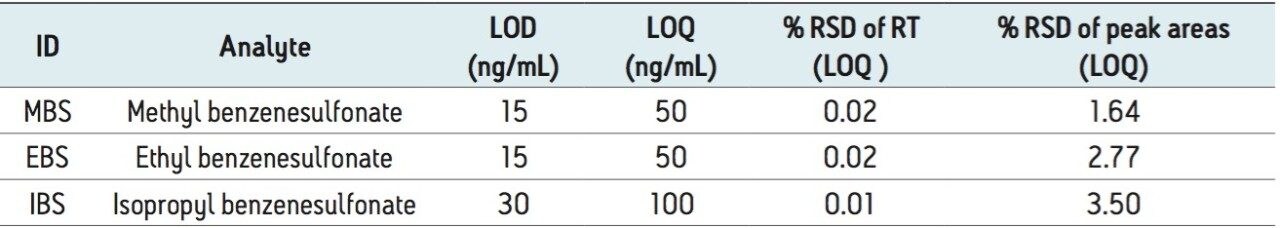
Limits of detection (LOD) and quantification (LOQ) were determined following the signal-to-noise criteria of 3:1 and 10:1, respectively. Data from six replicate injections of standard containing benzenesulfonate esters was evaluated to establish and to verify performance at the LOD and LOQ limits. The LOD and LOQ values for the alkyl sulfonate esters with UV detection are listed in Table 4. The LOQ values for both methyl and ethyl benzenesulfonates and for isopropyl benzenesulfonates was found to be 50 ng/mL and 100 ng/mL respectively.
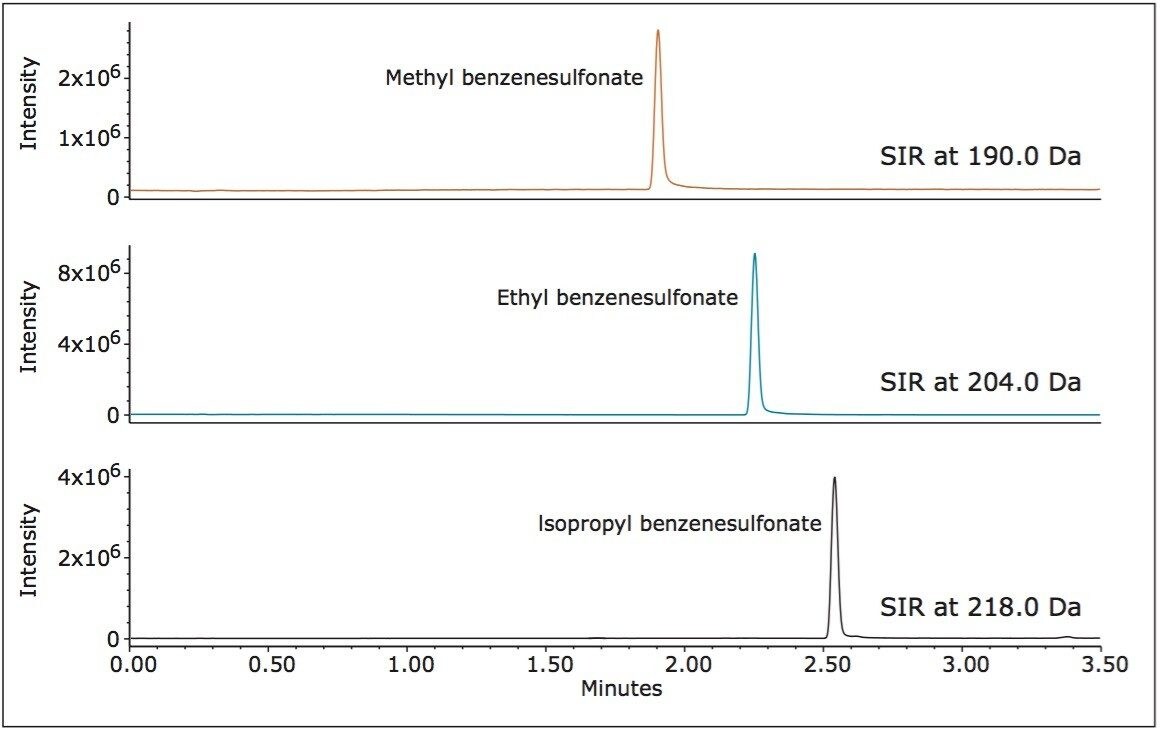
Under ammonium acetate mobile phase conditions, methyl, ethyl, and isopropyl esters of benzenesulfonic acid form ammonium adducts, [M+NH4].+ These ions were measured using single ion recording (SIR) mode, which determines the intensity for a single ion of interest (Figure 3). For targeted assay testing, the SIR mode enhances method sensitivity, and simplifies analysis and quantification.
To optimize sensitivity for methyl ester, different ammonium acetate concentrations ranging from 1 to 20 mM were investigated. It was observed that 1 mM ammonium acetate resulted in the highest MS response. In addition, increasing injection volume from 8 to 10 μL further improved sensitivity for methyl ester.
The method linearity with mass detection evaluated for methyl, ethyl, and isopropyl benzenesulfonates over 1.5 to 500 ng/mL was acceptable with the correlation coefficients (R2) of ≥0.9984 (Figure 4).
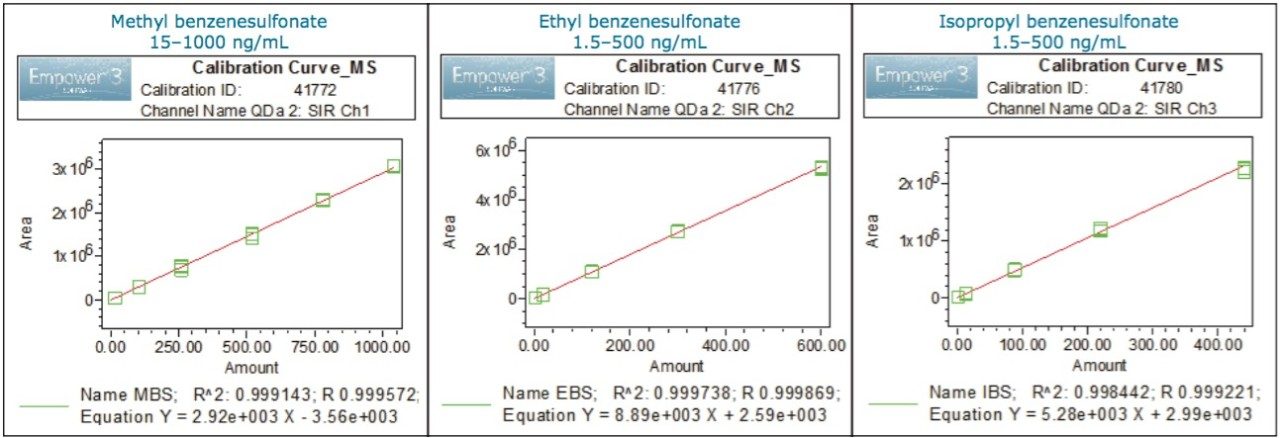
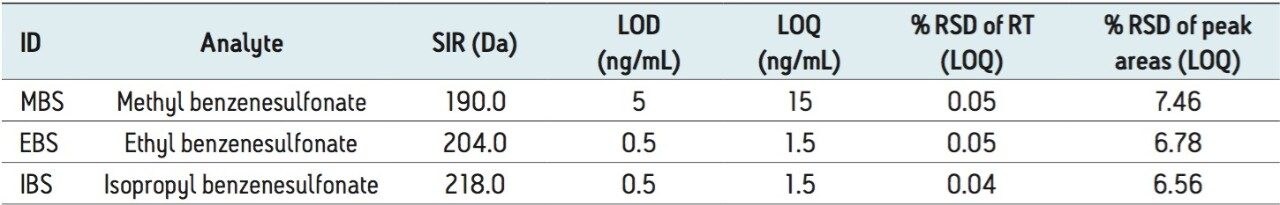
The LOD and LOQ were determined using the signal-to-noise data generated from six replicate injections of a standard containing benzenesulfonate esters. The LOQ and LOD values for MS detection are listed in Table 5. The LOQ for methyl benzenesulfonate and for both ethyl and isopropyl benzensulfonates was found to be 7.5 ng/mL and 1.5 ng/mL respectively.
Repeatability of the retention times and peak areas for six replicate injections of the LOQ solution were excellent for all three benzenesulfonate esters with % RSD of less than 0.05% for RT and 7.67% for peak areas respectively.
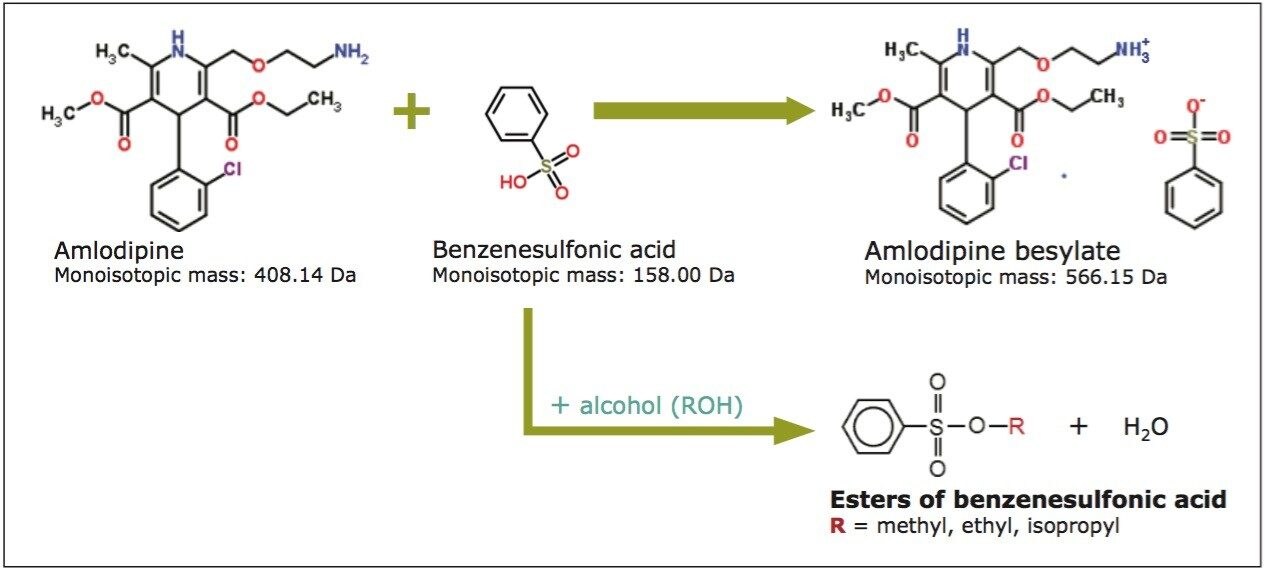
Amlodipine besylate API is the besylate salt of amlodipine and is used to treat high blood pressure (hypertension), chest pain (angina), and other conditions caused by coronary artery disease.5 It is available as tablets for oral administration with 2.5, 5, and 10 mg of drug substance per tablet, with the recommended maximum dosage being 10 mg once a day. During salt formation of amlodipine besylate API, benzenesulfonic acid may react with residual alcohols to form potentially genotoxic alkyl esters (Figure 5). Therefore, it is essential that this drug substance is monitored for the presence of these compounds to ensure safety of the pharmaceutical product.
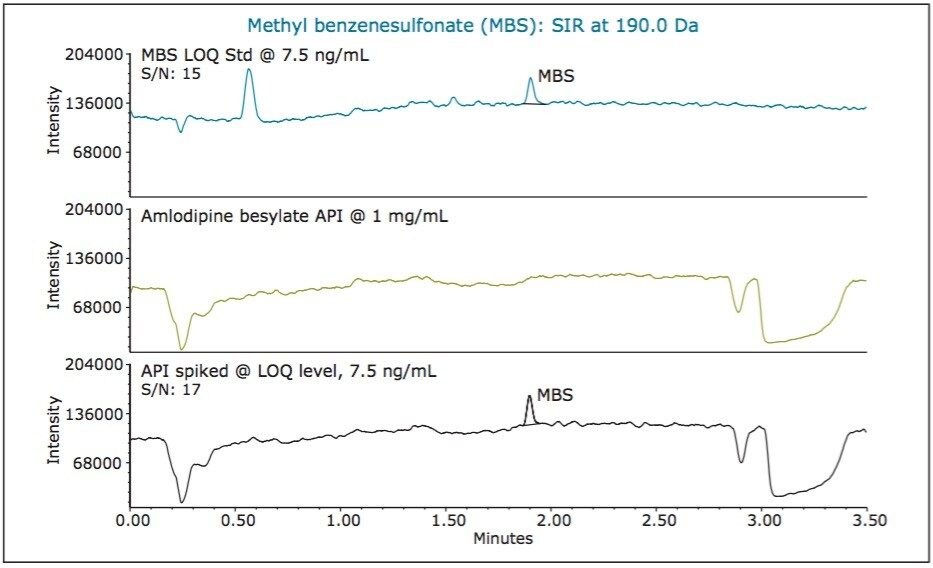
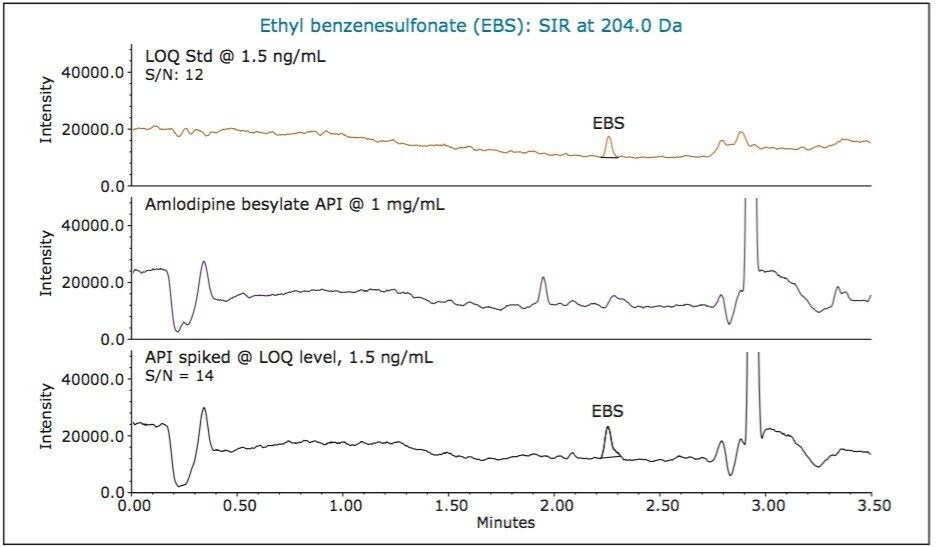
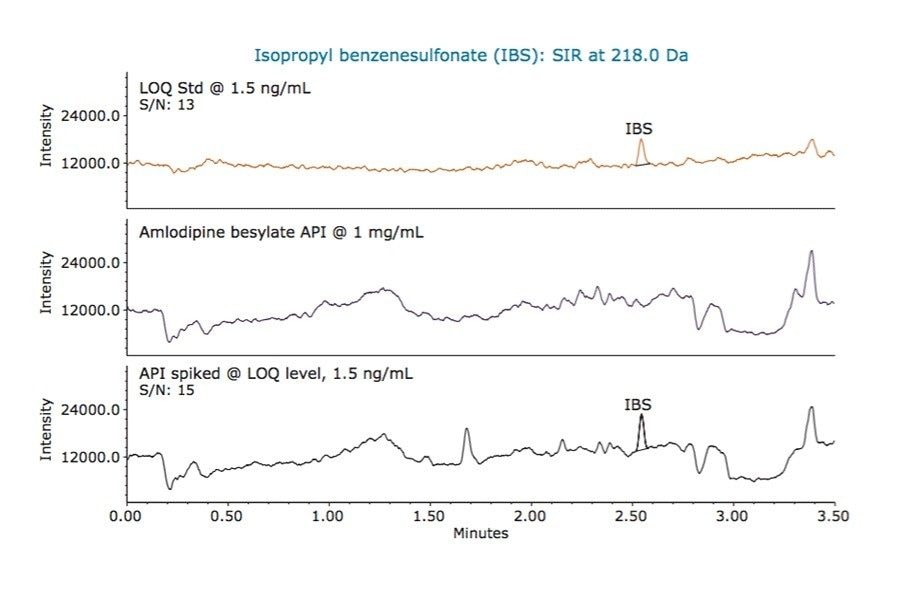
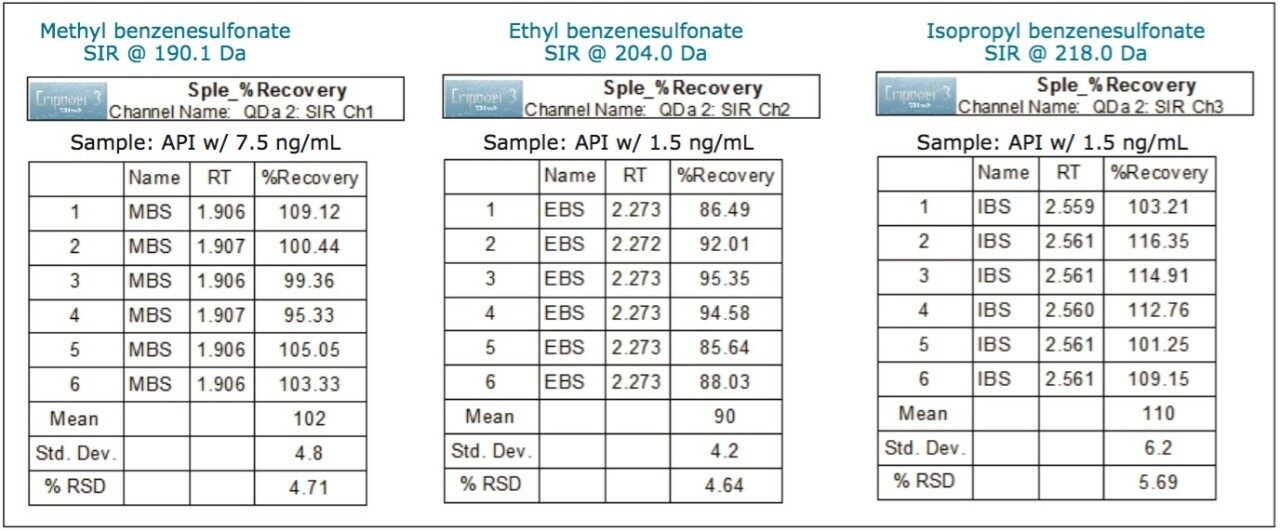
For low levels or trace analysis, it is important to show that the impurities can be separated and accurately measured in the presence of the sample matrix. To demonstrate specificity of our MS method, we spiked amlodipine besylate API with the three alkyl esters of benzenesulfonic acid at the LOQ levels. A sample containing 1 mg/mL of API was spiked with 7.5 ng/mL of methyl benzenesulfonate and 1.5 ng/mL of ethyl and isopropyl benzenesulfonates, respectively. The MS chromatographic data of the LOQ standard, unspiked API sample, and API sample spiked with alkyl esters is displayed in Figure 6, 7, and 8, respectively. The data show that the unspiked amlodipine besylate sample does not contain any peaks eluting at the retention times of the alkyl esters. The data also show that the signal-to-noise ratios for alkyl esters were comparable for the LOQ standards and spiked drug substance samples. Moreover, the average recovery of the analytes from the spiked API samples at the LOQ levels ranged from 90 to 110% with % RSD of 4.71 to 5.69 for recoveries of six sample injections (Figure 9). This demonstrates that the methyl, ethyl, and isopropyl benzenesulfonates can be accurately measured in presence of amlodipine besylate API.
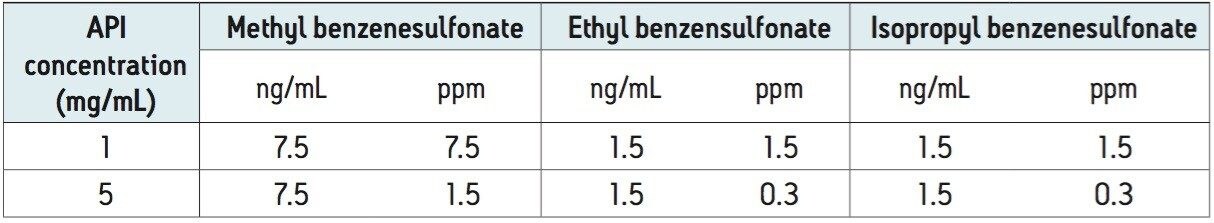
As discussed in the introduction, genotoxic impurities are typically reported in ppm relative to the API dose in a pharmaceutical product. This means that for our method, the MS quantification limit of 7.5 ng/mL for methyl benzenesulfonate corresponds to 7.5 ppm with respect to 1 mg/mL of API in a solution. This limit can also be accurately measured in the presence of 5 mg/mL API in a solution, which corresponds to 1.5 ppm. This is lower than the previously established value of 15 ng/mL, which is reported in 0.33 ppm based on an API sample concentration of 50 mg/mL.6 Table 6 shows the quantification limits for methyl, ethyl, and isopropyl benzenesulfonates calculated in ppm, based on a sample solution containing 1 and 5 mg/mL of API.
The ACQUITY UPLC H-Class System, coupled with the ACQUITY UPLC PDA and ACQUITY QDa detectors, provides an excellent solution for monitoring potential genotoxic impurities with accurate confirmation and quantitation.
The ACQUITY QDa enables quick and accurate determination of peak identity by mass detection. The use of the mass detector and single ion recording (SIR) enhances specificity and sensitivity of the UPLC method required for analysis of low level impurities in the pharmaceutical products. The reproducibility and accuracy of the MS method at the limit of quantification levels were excellent, demonstrating that mass detection is suitable for routine monitoring of genotoxic impurities in pharmaceutical products. In addition, the UPLC with mass detection method showed acceptable limits of quantification in the presence of 1 to 5 mg/mL of API to meet the regulatory requirements for genotoxic impurity analysis.
Overall, the ACQUITY QDa Detector is a robust and simple-to-use detector that can be added as an orthogonal detection technique to UV detection. It provides accurate and reliable results, making this technology ideal for routine testing of pharmaceutical products in the QC laboratory.
720005680, April 2016