
For forensic toxicology use only.
This application note details the extraction of THC-OH, THC-COOH, and THC from oral fluid samples using a novel SPE sorbent, Oasis PRiME HLB, in a μElution format for forensic toxicology applications.
This sorbent enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow. In addition, the μElution format enabled the direct injection of extracts without evaporation or reconstitution, minimizing the risk of nonspecific binding and sample losses. The unique nature of oral fluid resulted in some ion suppression that was not seen in other matrices. In order to overcome this signal suppression, a CORTECS C18 Column was utilized for high efficiency and the SPE wash step was optimized with the addition of 5% strong ammonia. These changes resulted in a method with negligible matrix effects (<10%) and calibration curves R2 value all greater than 0.999.
In conclusion, Oasis PRiME HLB has been successfully used to achieve consistent recoveries with minimal matrix effects as well as accurate quantification 4 orders of magnitude from oral fluid samples.
Cannabis continues to be a highly abused recreational drug. The increasing number of states legalizing it for medical use combined with the trend towards legalization for recreational purposes, means that there is a growing need for analytical methods for the quantification of Δ-9-tetrahydrocannabinol (THC), its metabolites including the active metabolite 11-hydroxy Δ-9-THC (THC-OH) and non-active metabolite 11-nor-9-Carboxy-Δ-9-THC (THC-COOH).1 While urine has traditionally been used to assess cannabis use, oral fluid has become increasingly popular as a matrix. Collection of oral fluid is relatively easy to perform, non-invasive and can be achieved under close supervision. Moreover, drug and metabolite concentrations in oral fluid provide better indications of current impairment than urine concentrations, so there is a higher probability that the subject is experiencing pharmacological effects at the time of sampling.2,3 The cut off level for THC use was reported as 2 ng/mL in oral fluid,5 which means any analytical method should be able to accurately quantify at this concentration.
This method details the extraction and analysis of THC and its major metabolites, 11-THC-OH and 11-THC-COOH from oral fluid using the Oasis PRiME HLB µElution Plate, followed by UPLC-MS/MS analysis. The SPE procedure is simple and very efficient, with elution in LC compatible solvents, allowing for direct injection, without evaporation and reconstitution of samples. Analysis is rapid with all analytes eluting in 3 minutes. Recoveries were excellent (all greater than 75% with %RSDs <6) and matrix effects were minimal (<10% ME) for all compounds. Quantitative results were highly reproducible. All calibration curves were linear and R2 values were greater than 0.999. Quality control results were within 10% of expected concentrations with average %RSDs less than 3%.
|
UPLC system: |
ACQUITY I-Class UPLC System |
|
Column: |
CORTECS UPLC C18 Column 1.6 μm, 2.1 x 100 mm |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Mobile phase A (MPA): |
Water with 0.1% formic acid |
|
Mobile phase B (MPB): |
ACN with 0.1% formic acid |
|
Strong wash solvent: |
70:30 ACN:Water with 2% formic acid |
|
Weak wash solvent: |
10% ACN |
|
Injection volume: |
5 μL |
|
The gradient ramp is shown in Table 1. |
|
Time (min.) |
Flow (mL/min.) |
%A |
%B |
|---|---|---|---|
|
0.0 |
0.6 |
50 |
50 |
|
1.0 |
0.6 |
50 |
50 |
|
3.0 |
0.6 |
5 |
95 |
|
5.0 |
0.6 |
5 |
95 |
|
5.6 |
0.6 |
50 |
50 |
|
6.0 |
0.6 |
50 |
50 |
|
MS system: |
Xevo TQ-S Mass |
|
Spectrometer Ionization mode: |
ESI Positive |
|
Capillary voltage: |
2.0 kV |
|
Cone voltage: |
Optimized for each analyte |
|
Cone gas: |
150 L/hr |
|
Desolvation temp.: |
500 °C |
|
Source temp: |
150 °C |
All standards and stable isotope labelled internal standards were purchased from Cerilliant (Round Rock, TX, USA). Stock standards at 100 µg/mL were prepared in 40% methanol (THC, THC-OH and THC-COOH). A working internal standard solution, consisting of 100 ng/mL THC-D3, THC-OH-D3 and THC-COOH-D3 was also prepared in 40% methanol. Individual calibrators and quality control standards were prepared daily in 40% methanol. 200 µL of each working calibrator or QC standard was added to 1800 µL of oral fluid to make calibration curves and QC samples. Calibrator concentrations ranged from 0.05–100 ng/mL for all analytes. Quality control samples were prepared at 0.375, 1.75, 7.5 and 37.5 ng/mL, in oral fluid.
Oral fluid samples were collected with Quantisal collection device from Immunalysis according to the manufacturer’s directions. The collection applicator was saturated with oral fluid (spiked), and then placed in a collection vial, which contained 3.0 mL of sample stabilization buffer. Per Quantisal instruction, this was claimed to be the equivalent of collecting 1.0±0.1 mL of sample. 1 mL acetonitrile was then added to the collection vial to help improve extraction. The collection kit was stored in a refrigerator overnight to simulate the transit time of the sample and to allow for complete equilibration between the sample in the pad and the stabilization buffer mix in the collection vial.
500 µL aliquots of buffer stabilized oral fluid samples (equivalent to 100 µL oral fluid) were pre-treated by adding 200 µL 4% H3PO4 and 10 µL of working IS mixture (100 ng/mL in 40% MeOH).
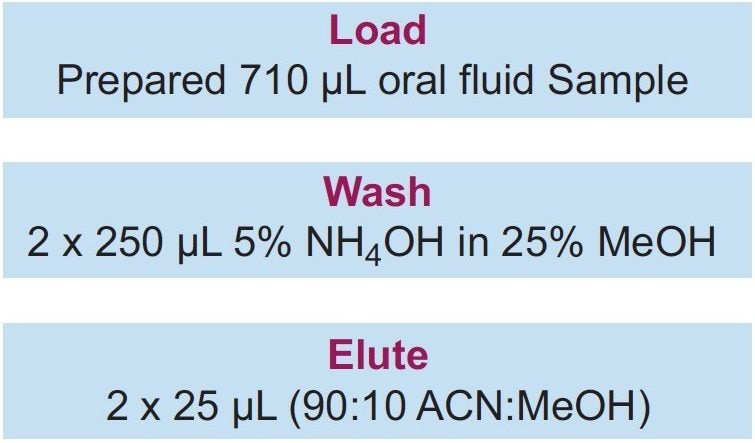
The entire pre-treated sample (total of 710 µL) was directly loaded on to the Oasis PRiME HLB µElution Plate without conditioning or equilibration, followed by washing with 2 x 250 µL 5% NH4OH in 25:75 methanol:water. All the wells were then eluted with 2 x 25 µL 90:10 ACN:MeOH and diluted with 50 µL of water. 5 µL was injected onto the UPLC-MS/MS system. The SPE extraction procedure is summarized in Figure 1.
Analyte recovery was calculated according to the following equation:
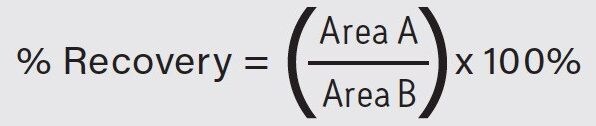
Where A equals the peak area of an extracted sample and B equals the peak area of an extracted blank matrix sample in which the compounds were added post-extraction.

The peak area in the presence of matrix refers to the peak area of an extracted matrix sample in which the compounds were added post-extraction. The peak area in the absence of matrix refers to analytes in a neat solvent solution.
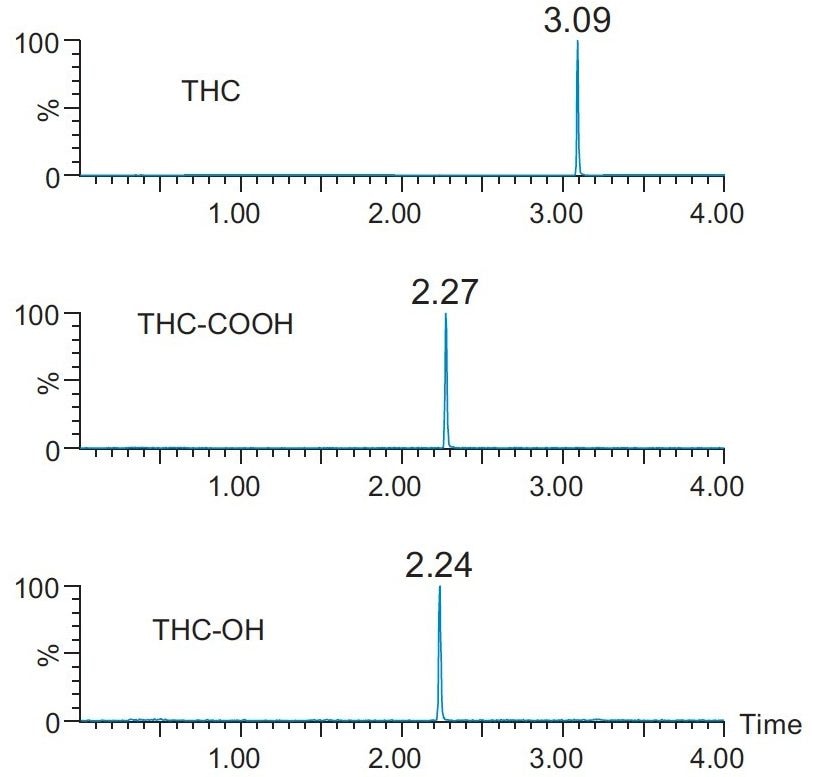
A representative UPLC chromatogram of the three cannabinoids from an extracted calibrator at 1 ng/mL is shown in Figure 2. Using a CORTECS UPLC C18 Column, all compounds eluted in 3 minutes. Peak shape was excellent for all compounds with all peak widths were under 1.8 seconds at 5% of baseline.
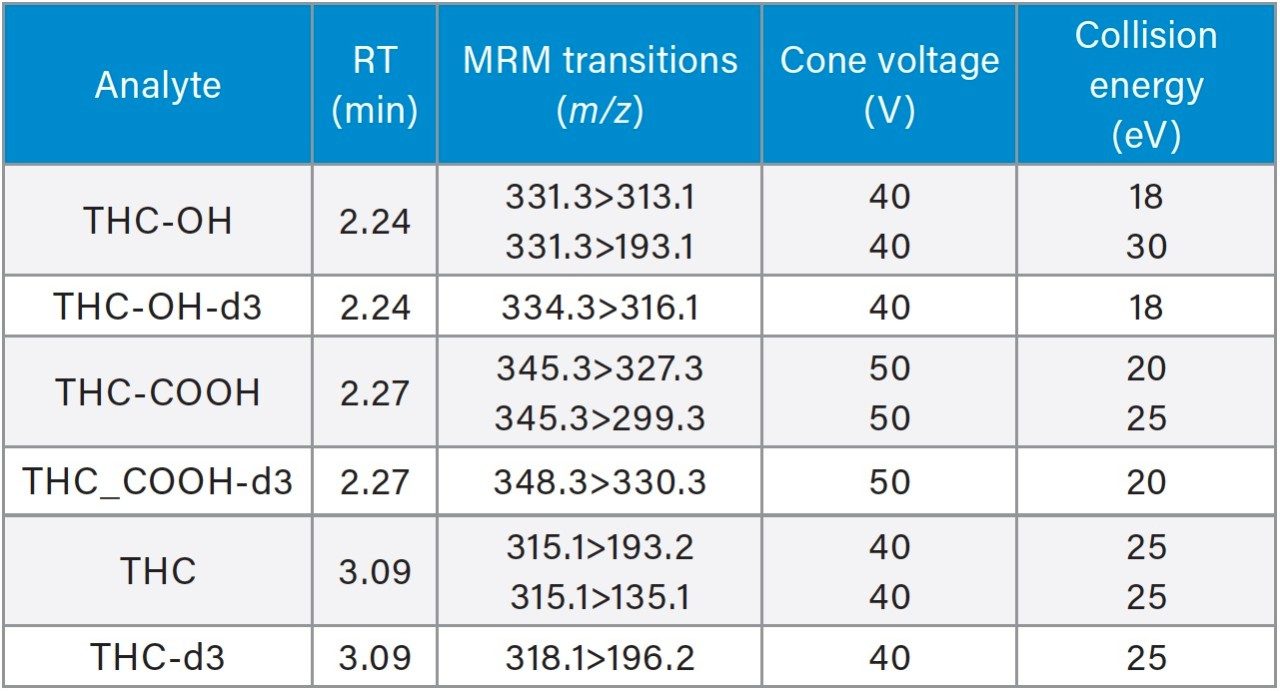
Table 2 lists the UPLC separation retention time and individualized MS parameters of the cannabinoids and their stable isotope labelled internal standards, including MRM transitions, cone voltage, and collision energy. Two MRM transitions were used for each compound, a primary (listed first) and a confirmatory transition (listed second).
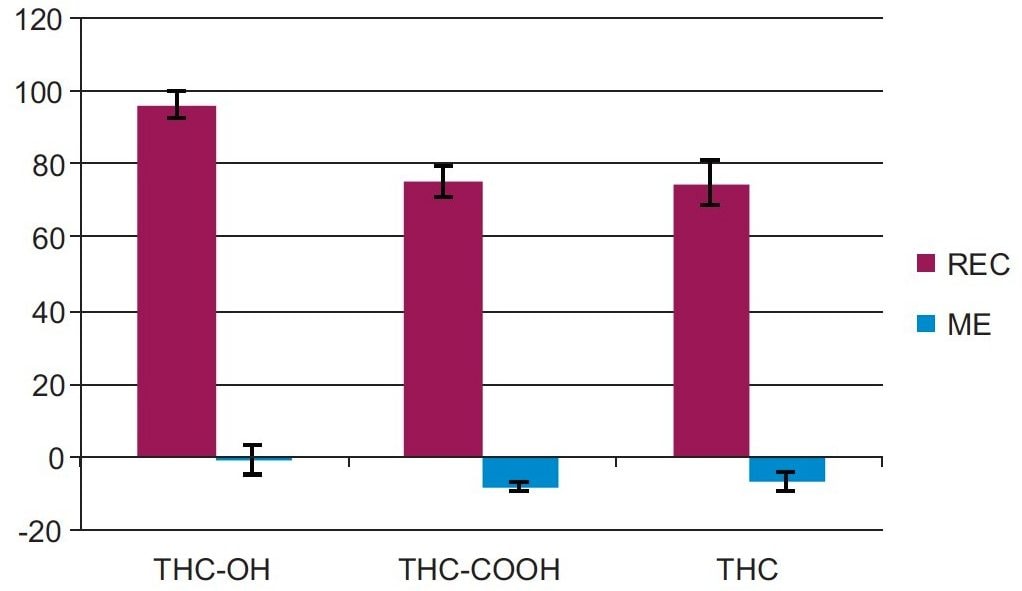
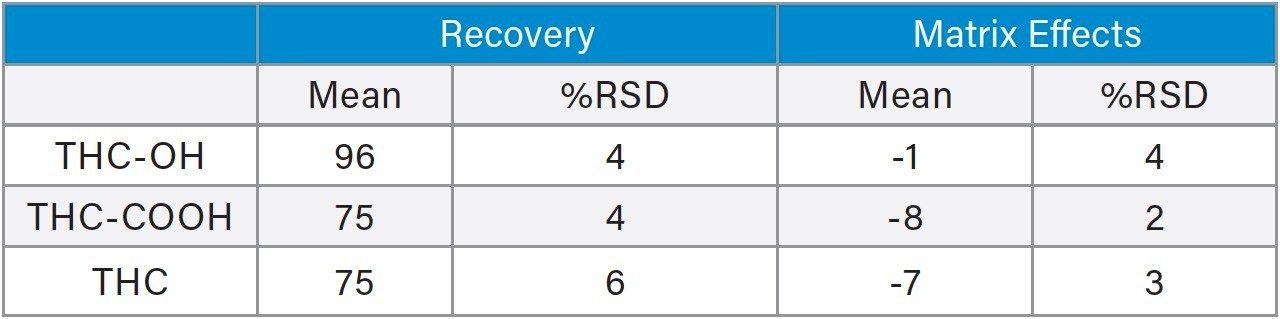
Extraction recoveries were high and consistent. As Figure 3 shows, recovery for all analytes was at least 75% with all %RSDs within 6% demonstrating the reproducibility of Oasis PRiME HLB. Matrix effects were minimal, at less than 10% for all compounds. Once again, the very low standard deviations (6% or less) demonstrate the consistency of extraction and cleanup seen with Oasis PRiME HLB. All recovery and matrix effect data are summarized in Table 3. The SPE wash step required optimization to eliminate suppression from the oral fluid matrix. The addition of 5% strong ammonia to the wash solution minimized the suppression, resulting in the near complete elimination of matrix effects.
Calibration and quality control samples were prepared as previously described in the materials and methods section. Calibration ranges were from 0.1–100 ng/mL for THC-OH and THC-COOH, and 0.05–100 ng/mL for THC. Quality control samples were prepared at low, medium, and high concentrations as appropriate for the calibration ranges.
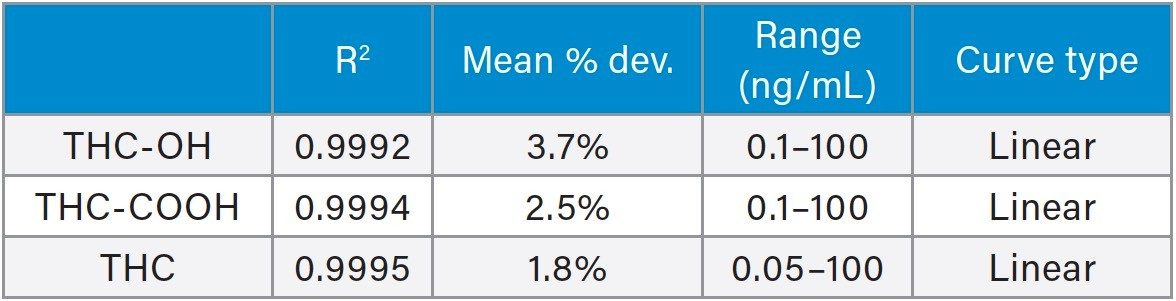
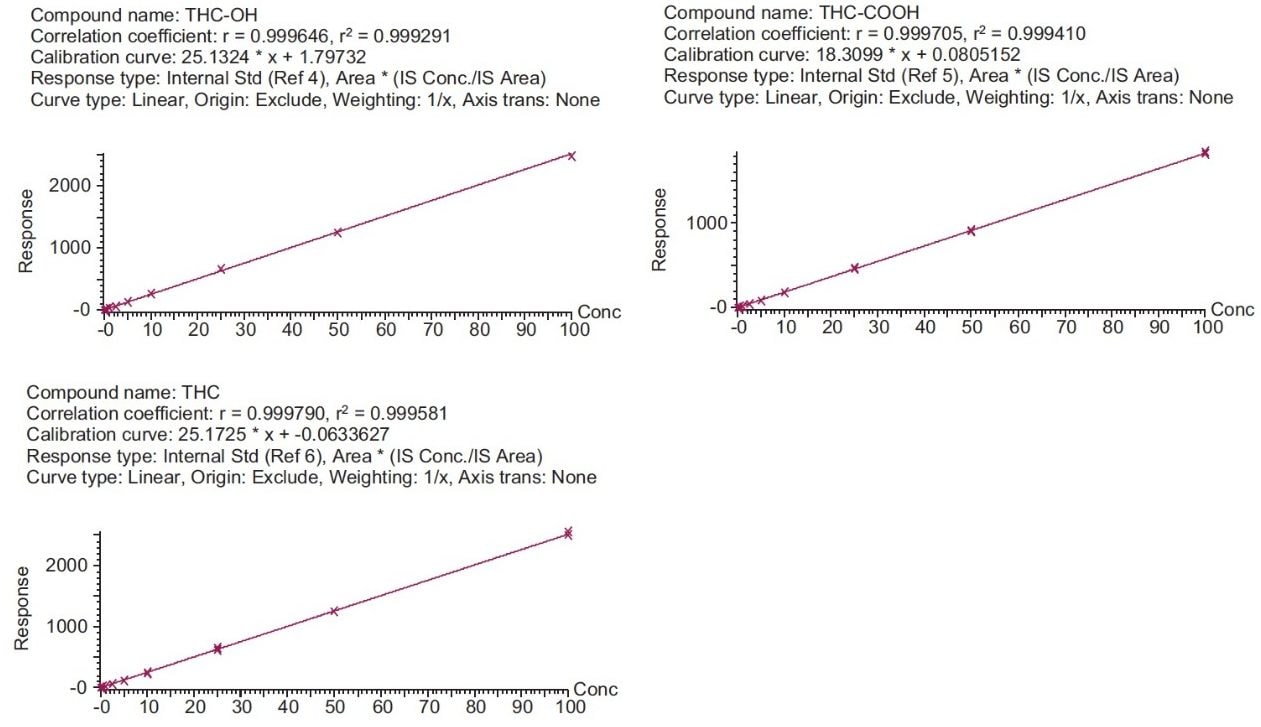
Calibration and quality control (QC) results indicate that this method is linear, accurate and precise. All compounds had linear responses over the entire calibration range with R2 values of 0.999 or greater using 1/x weighting. Figure 4 shows the calibration curves and Table 4 summarizes the data from these curves for all the compounds. Lower limits of quantification (LLOQ) were 0.1 ng/mL for THC-OH and THC-COOH and 0.05 ng/mL for THC. In each case, all FDA recommendations for accuracy, precision, linearity and analytical sensitivity were met for validated methods.4
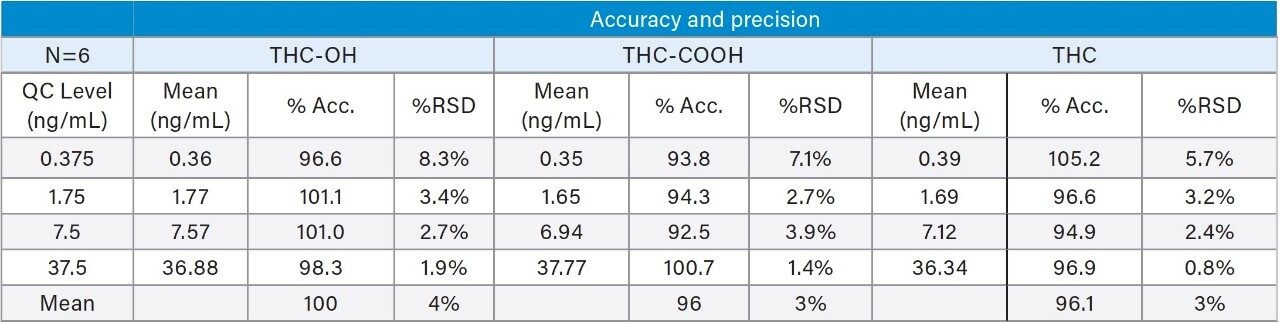
Quality control samples prepared at 0.375, 1.75, 7.5, and 37.5 ng/mL were accurate and precise. All QC values were within 10% of their target values, and most were within 5%. This data can be seen in Table 5. This demonstrates that the method is linear, accurate and precise over a calibration range that includes the entire scope of expected values of samples. The method was also proved to be both selective and sensitive enough to routinely measure THC in oral fluid well below 2 ng/mL cut off level. This was exemplified by the excellent accuracy and precision at the 0.375 ng/mL QC sample level, where calculated concentrations of all six replicates were within an average of 6% of expected.
This application note details the extraction of THC-OH, THC-COOH, and THC from oral fluid samples using a novel SPE sorbent, Oasis PRiME HLB, in a µElution format for forensic toxicology applications. This sorbent enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow. In addition, the µElution format enabled the direct injection of extracts without evaporation or reconstitution, minimizing the risk of nonspecific binding and sample losses. The unique nature of oral fluid resulted in some ion suppression that was not seen in other matrices. In order to overcome this signal suppression, a CORTECS C18 Column was utilized for high efficiency and the SPE wash step was optimized with the addition of 5% strong ammonia. These changes resulted in a method with negligible matrix effects (<10%) and calibration curves R2 value all greater than 0.999.
Recoveries were very consistent, with recoveries >75%, with RSDs under 6%, and minimal matrix effects for all compounds. Linearity, accuracy, precision and analytical sensitivities were excellent for all compounds. All accuracies were within 10% of target concentrations with average %RSDs less than 3% for QC samples, demonstrating the high reproducibility arising from the combination of this sorbent and the UPLC-MS/MS method. In conclusion, Oasis PRiME HLB has been successfully used to achieve consistent recoveries with minimal matrix effects as well as accurate quantification 4 orders of magnitude from oral fluid samples.
720005729, June 2016