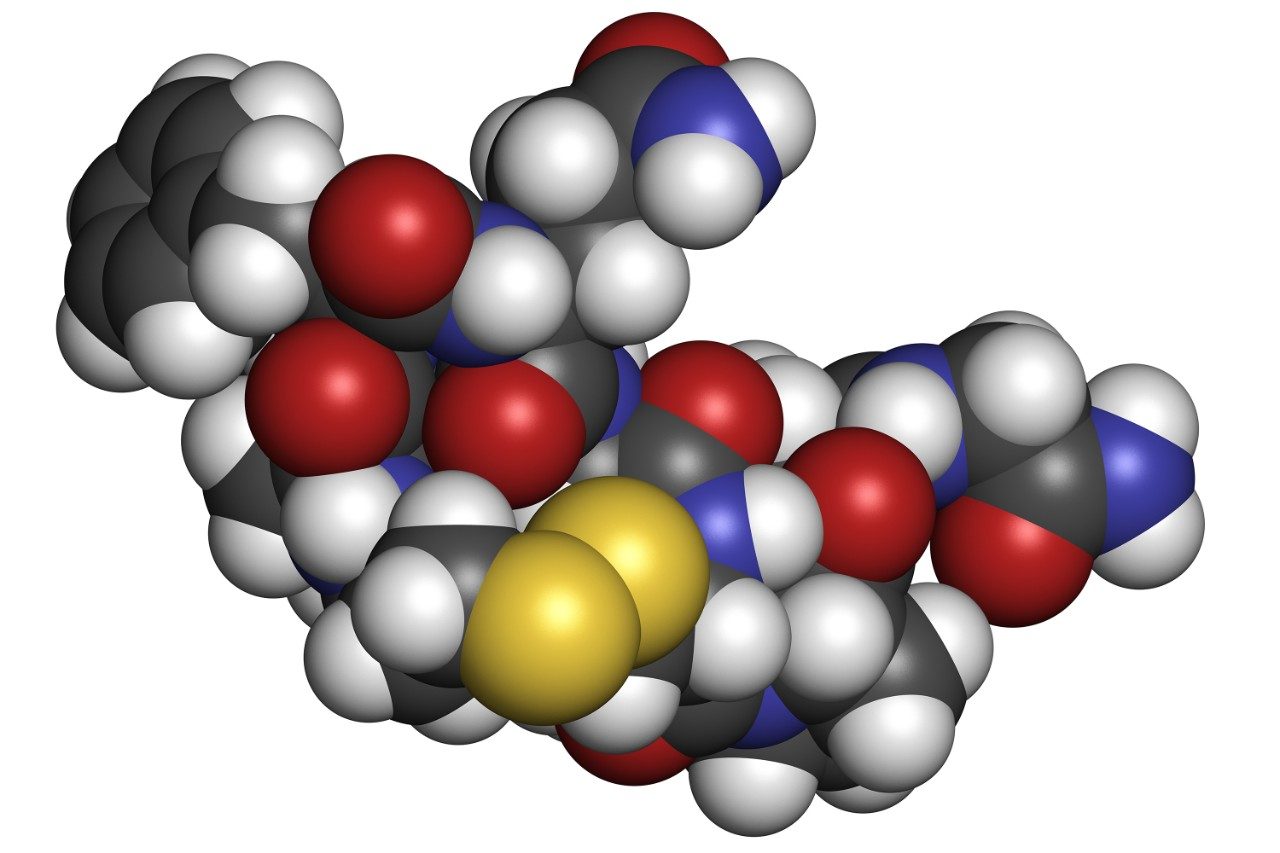
This application note investigates the improved sensitivity and decreased sample volume requirements for the therapeutic and endogenous cyclic peptides: desmopressin, vasopressin and octreotide.
The use of peptides and proteins as therapeutic agents has increased significantly in recent years. Thus, the demand for their analysis for toxicokinetic and pharmacokinetic studies is increasing as well.
Historically, biologics have been quantified using ligand binding assays (LBAs). However, with recent advances in mass spectrometry (MS) and liquid chromatography (LC) technologies current approaches towards peptide quantification in biological fluids now include LC-MS/MS. This is in part driven by the fact that LBAs can suffer from significant cross-reactivity issues and lack of standardization. Additionally, LC-MS/MS also has the advantage of greater accuracy and precision, broader dynamic ranges, specificity, and speed of method development. However, accurate quantification of peptides by LC-MS/MS is often not without its own challenges. Peptides have diverse pharmacokinetic profiles, often low circulating plasma levels (pg/mL), generally low MS sensitivity, and require chromatographic resolution from endogenous isobaric matrix interferences.1 Therefore, to achieve low pg/mL quantification limits, large plasma sample volumes (0.2–1 mL) and sample injection volumes are often required.2-6 These volumes are often impractical in discovery studies. Thus, the demand for quantitative bioanalytical assays that use decreased sample volumes, while maintaining or improving sensitivity are highly desired.
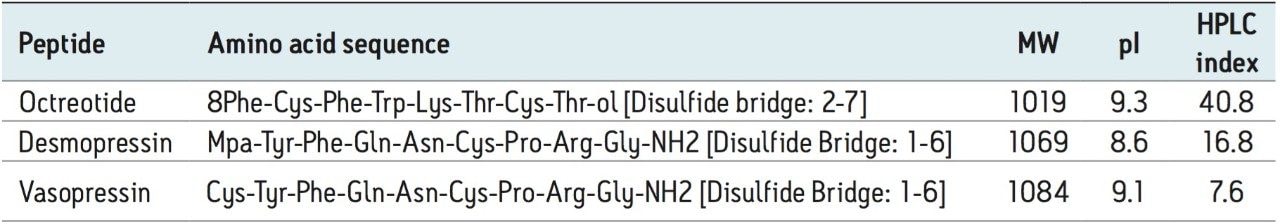
This application investigates the improved sensitivity and decreased sample volume requirements for the therapeutic and endogenous cyclic peptides: desmopressin, vasopressin and octreotide.7-9 The general properties of these peptides are shown in Table 1. Using a combination of selective μElution mixed-mode SPE sample preparation, optimal MS precursor and fragment choice, and the ionKey/MS System (source shown in Figure 1), limits of quantification of 1 pg/mL in plasma were achieved. Capitalizing on the attributes of the ionKey/MS System facilitated reducing plasma sample required to 25–100 μL.
|
LC system: |
ACQUITY UPLC M-Class, configured for trap and back-flush elution |
|
Separation device: |
iKey HSS T3, 1.8 μm, 100Å, 150 μm x 100 mm iKey (p/n 186007261) |
|
Trap column: |
ACQUITY UPLC M-Class Symmetry C18, 5 μm, 300 μm x 50 mm (p/n 186007498) |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Loading solvent: |
98:2 mobile phase A:B, 25 μL/min for first two minutes, reverse valve |
|
Valve position: |
Initial position one (forward loading of trap), switch to position two at two minutes (back flush elute of trap onto the analytical column) |
|
Analytical gradient: |
See Table 2 |
|
Elution flow rate: |
3.0 μL/min |
|
iKey temp.: |
75 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
5 μL |
|
Total run time: |
12.0 min |
|
Collection plates: |
Waters 1 mL Collection Plates |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.8 kV |
|
Source temp.: |
120 °C |
|
Cone gas flow: |
100 L/hr |
|
Collision cell pressure: |
5.5 x 10(-3) mbar |
|
Collision energy: |
Optimized by component, see Table 3 |
|
Cone voltage: |
Optimized by component, see Table 3 |
|
Chromatography software: |
MassLynx 4.1 |
|
Quantification software: |
TargetLynx |

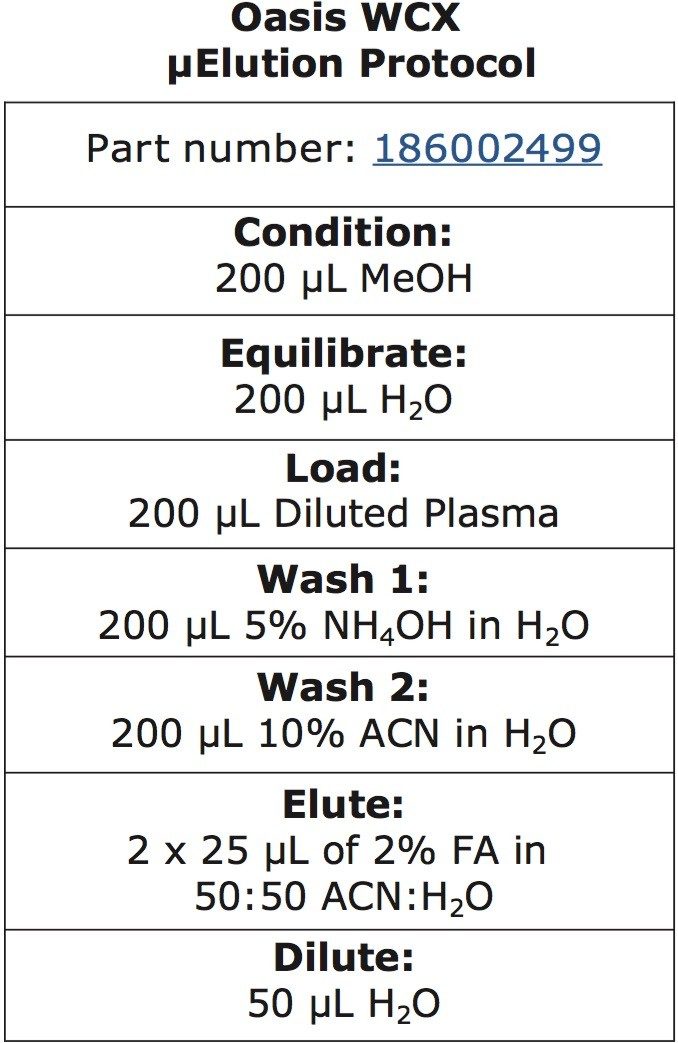
100 μL of human plasma was diluted 1:1 with 4% H3PO4 in water and mixed.
Pre-treated plasma samples were extracted according to the protocol in Figure 2. All solutions are made up by volume. All extraction steps were applied to all wells of the μElution plate that contained samples.
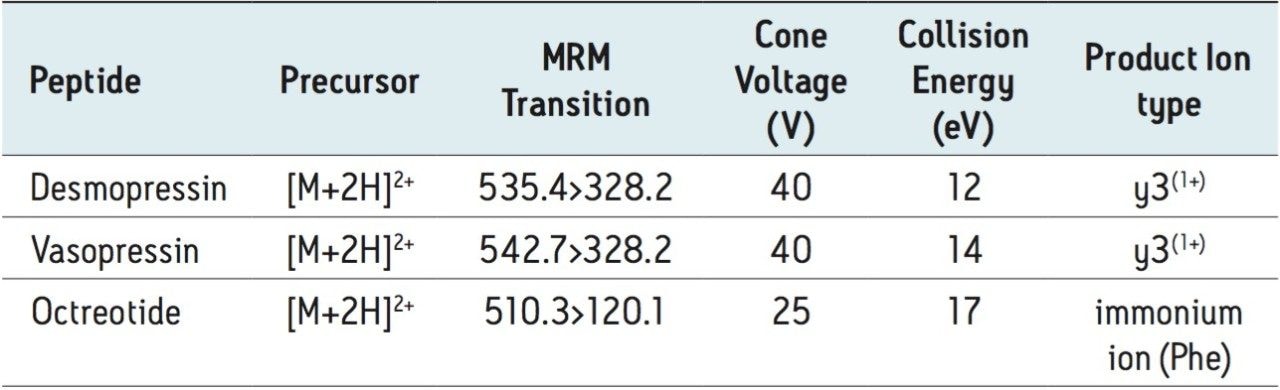
The 2+ precursors of desmopressin (m/z 535.45), vasopressin (m/z 542.75), and octreotide (m/z 510.30) were used for quantitation. Their corresponding fragments and optimal MS conditions are shown in Table 3. In this assay, the use of highly specific b/y ion specific fragments was more challenging due to the small size and cyclic nature of these peptides. The fragment at m/z 328.2, corresponding to a y31+ ion, was chosen for desmopressin and vasopressin. The fragment at m/z 120.1, corresponding to the phenylalanine immonium ion, was used for octreotide.
Chromatographic separation was achieved using the novel microfluidic chromatographic iKey Separation Device. The iKey Separation Device (Figure 3) is packed with UPLC-grade sub-2-µm particles that permit operation at high pressure and results in highly efficient LC separations. By integrating microscale LC components into a single platform design, problems associated with capillary connections, including manual variability, leaks, and excessive dead volume, are avoided. Use of the iKey HSS T3, 1.8 µm, 100Å, 150 µm x 100 mm (p/n 186007261) provided chromatographic retention, excellent peak shape, narrow peak widths (<4.5 seconds at base), and resolution from endogenous matrix interferences.

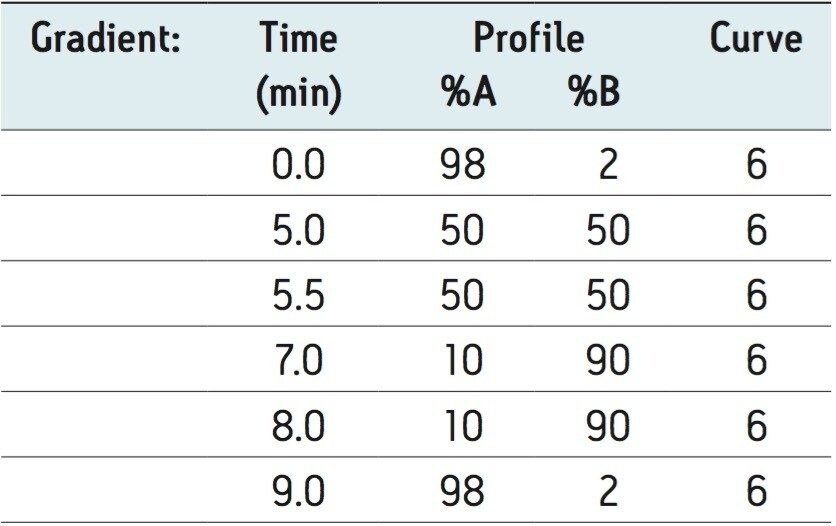
The peptides were eluted using a linear gradient from 2–50% B over 5 minutes, Table 2. Representative chromatograms are shown in Figure 4. The use of a trap and back-flush elution strategy, provided further sample cleanup and facilitated the loading of 5 μL of the high organic SPE eluate (required to maintain solubility of the peptides) without experiencing analyte breakthrough. Additionally, the ability to inject sample volumes typical for 2.1 mm analytical scale LC analysis on the iKey Separation Device can provide the substantial gains in sensitivity that are often required to accurately and reliably detect low pg/mL levels of peptides and proteins in complex matrices.
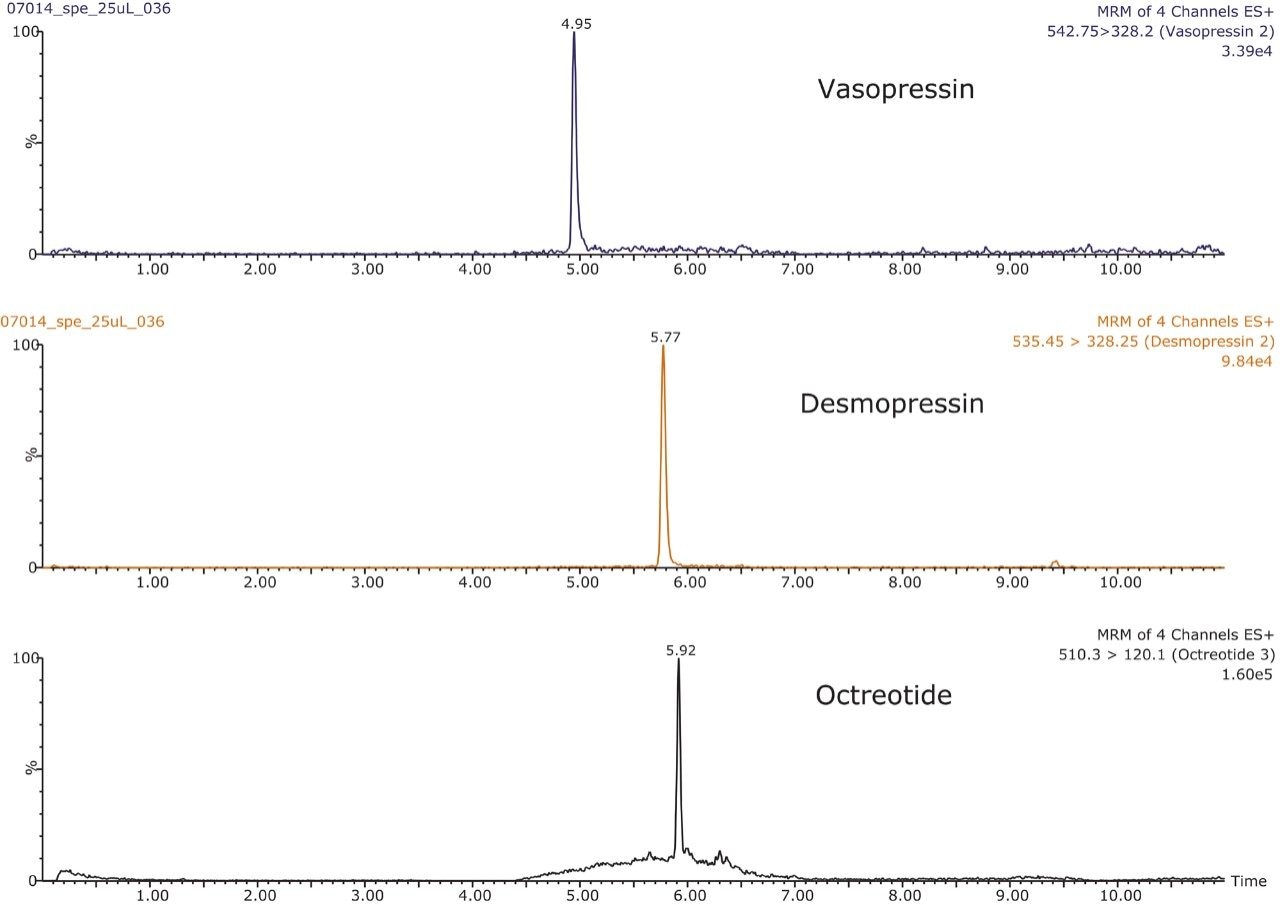
Versus analytical scale (2.1 mm I.D.), the ionKey/MS System generally offers increased sensitivity, making it ideal for high sensitivity peptide analysis. This also facilitates the use of smaller sample volumes whilst maintaining or improving sensitivity. In Figures 5 and 6, detection of 2.5 pg/mL of desmopressin and octreotide was easily obtained from extraction of 25, 100, or 200 μL human plasma, using injection volumes ≤10 μL.
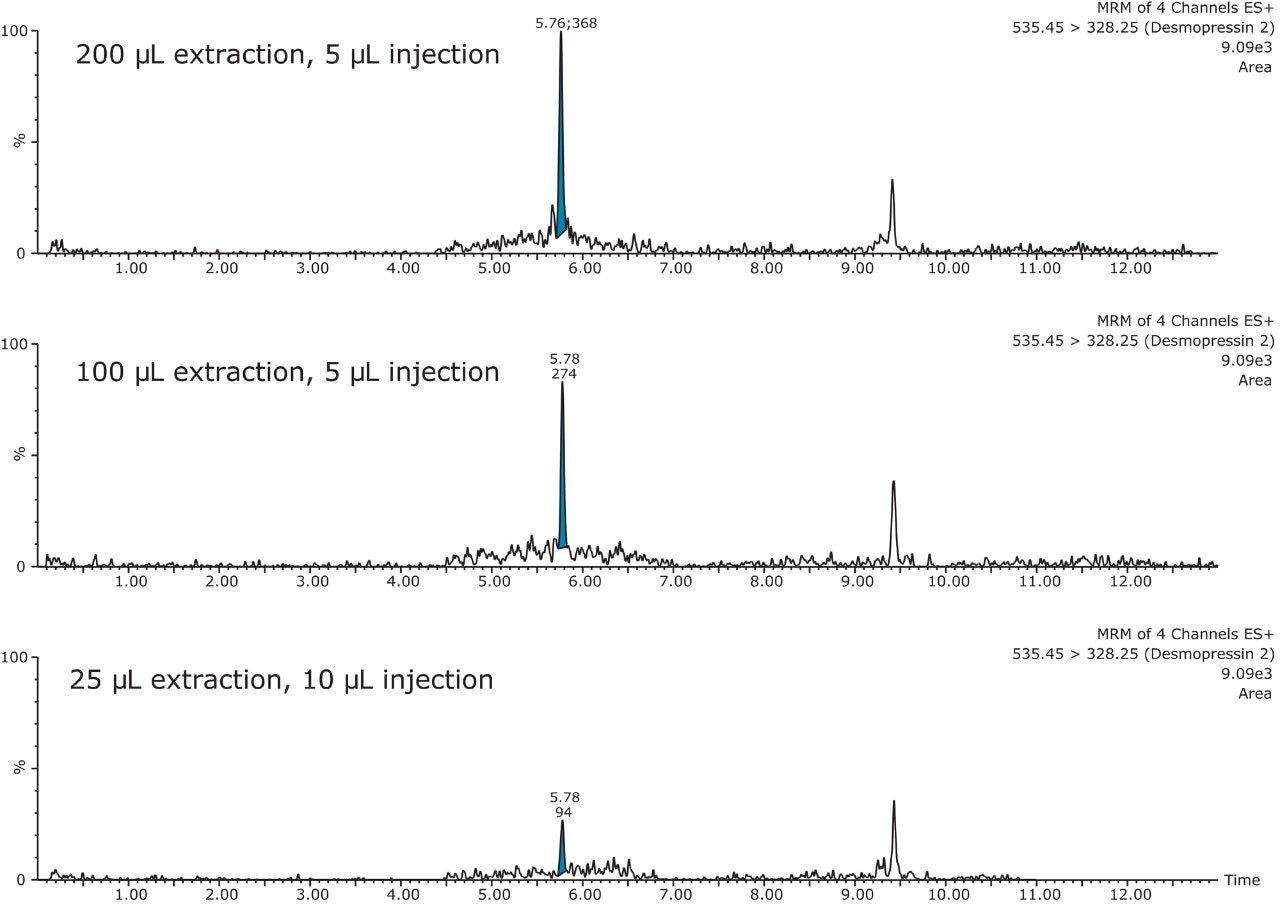
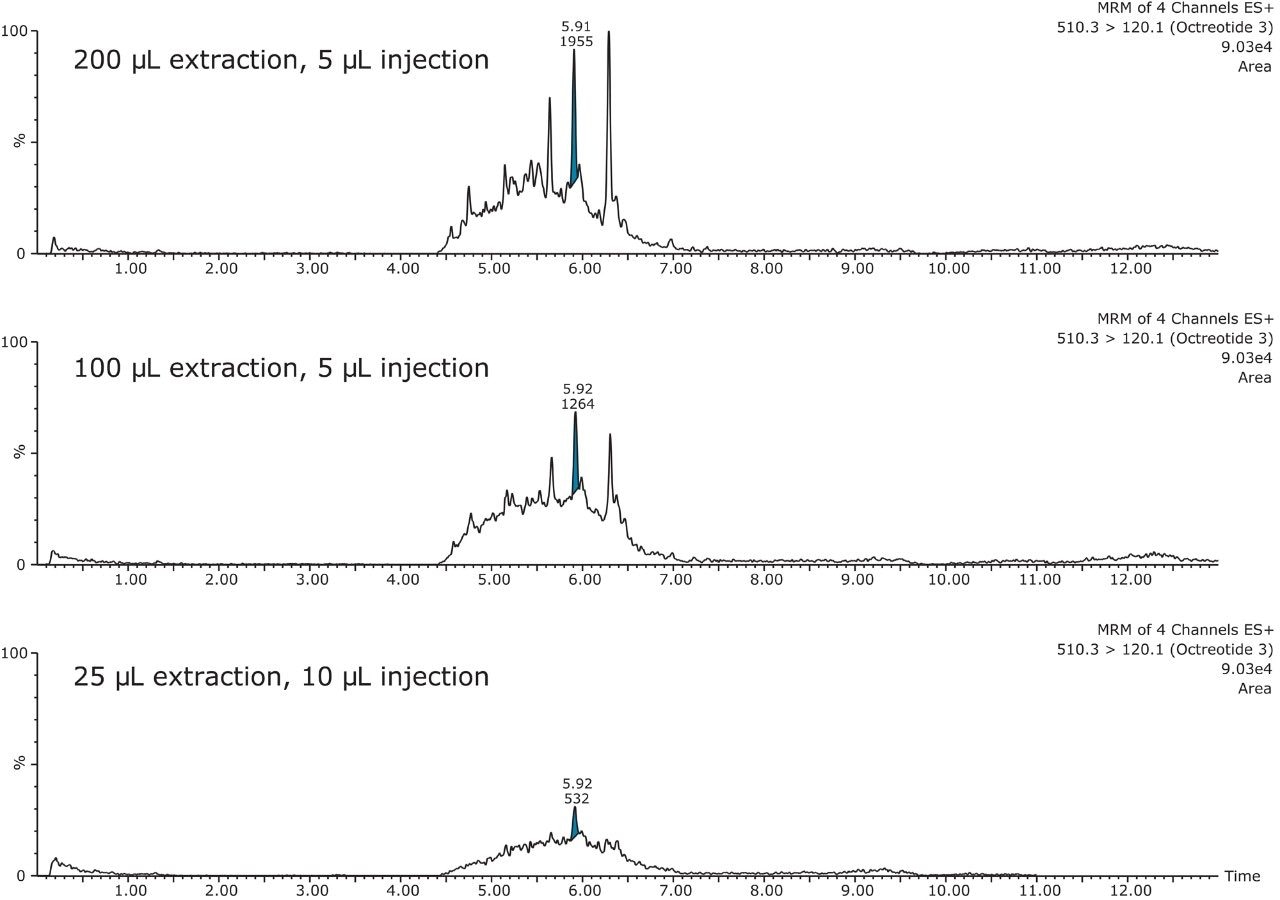
SPE was performed using Oasis WCX, which has both reversed-phase and ion-exchange modes of retention. The orthogonality introduced by the use of mixed-mode sorbents such as these enables greater sample cleanup, improved selectivity, and the sensitivity required for these peptides. Briefly, desmopressin, vasopressin, and octreotide were spiked at various concentrations into the plasma and mixed. These samples were then acidified with 4% H3PO4, which helped disrupt protein binding and reduce sample viscosity, improving contact time with the sorbent. Samples were loaded to the SPE device, and washed with 5% NH4OH followed by 10% acetonitrile. The optimum elution solution was 50% organic, 25% water, with 2% formic acid.
The 96-well Oasis μElution Plate format facilitates fast sample processing (under 30 minutes), and is compatible with automation by most liquid-handling robotic systems, improving sample throughput. Additionally, this format also provides the ability to elute in very small sample volumes, minimizes the potential for adsorptive peptide losses, as well as concentrates the sample for increased sensitivity.
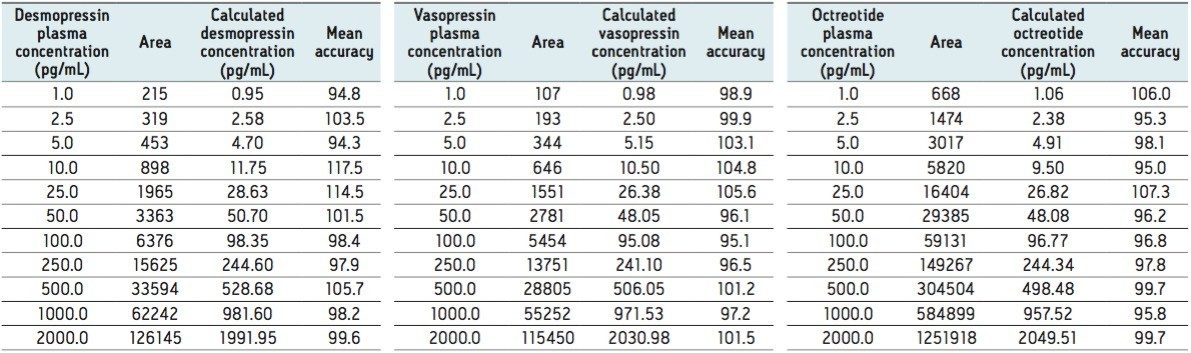
To generate standard curves, human plasma was fortified with desmopressin, vasopressin and octreotide at the following final concentrations: 1, 2.5, 5, 10, 25, 50, 100, 250, 500, 1000, and 2000 pg/mL. SPE of the fortified plasma samples was performed as described above. The calibration curves were constructed using peak areas of the calibration samples by applying a one/concentration (1/x) weighted linear regression model. Using 100 μL of plasma, calibration lines were obtained for each peptide and are shown in Figure 7, panels A, B, C.
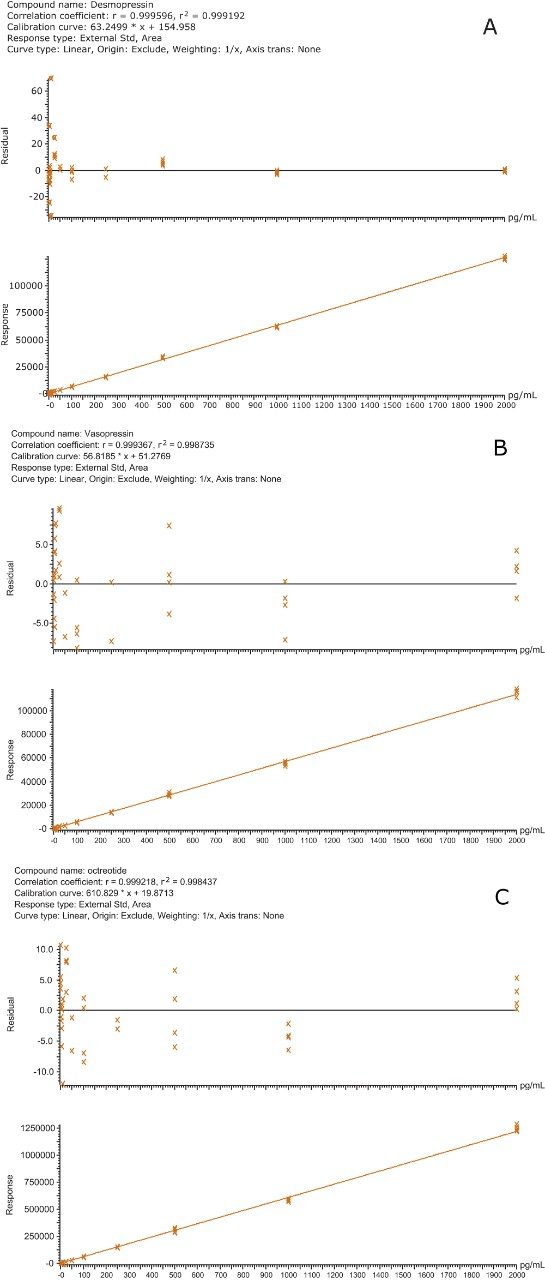
A summary of standard curve performance is shown in is shown in Table 4. Using 1/X regressions, quantification was linear from 1–2,000 pg/mL with R2 values of >0.99 for all 3 peptides monitored. Representative chromatograms for extracted desmopressin, vasopressin, and octreotide plasma standard samples are shown in Figures 8–10.
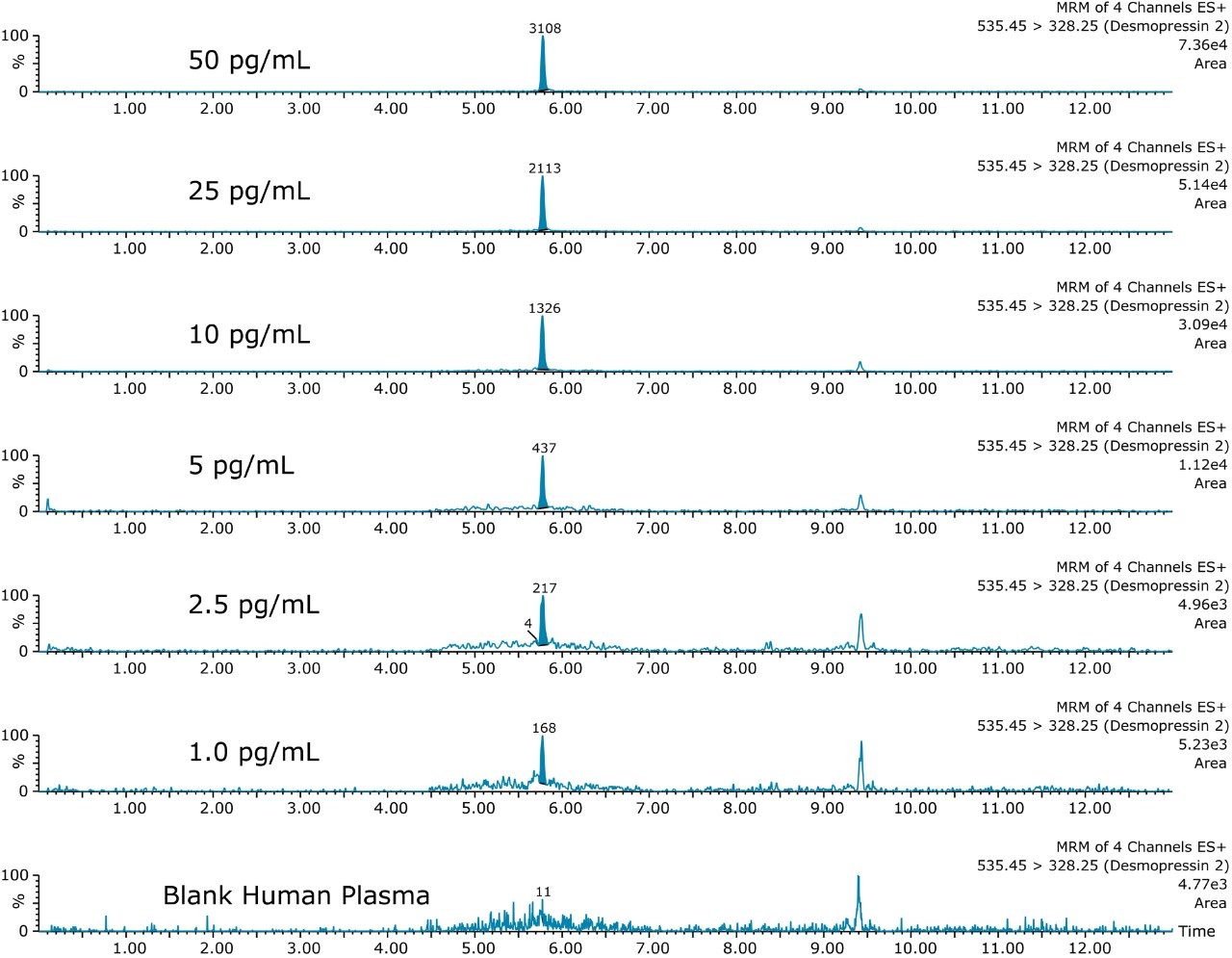
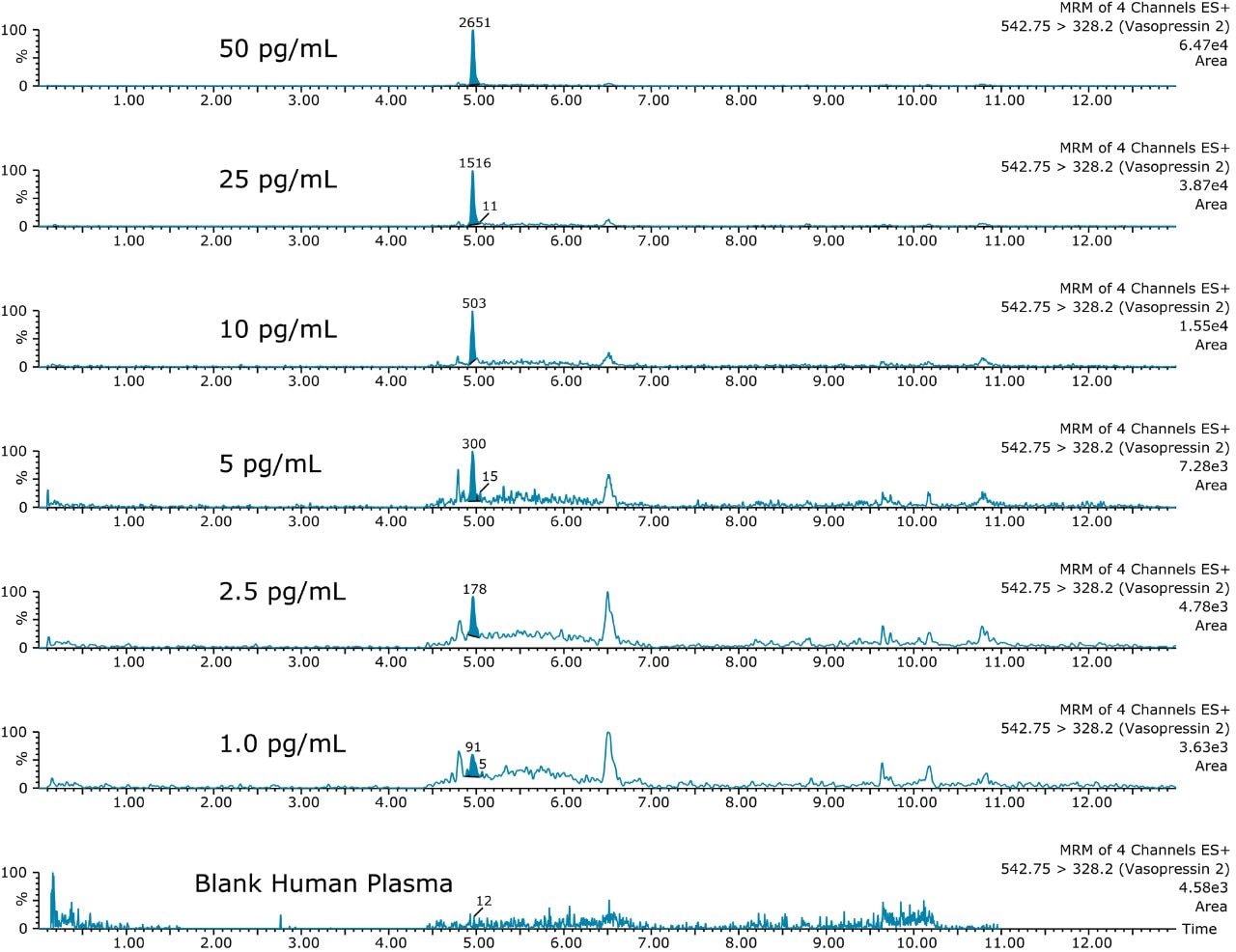
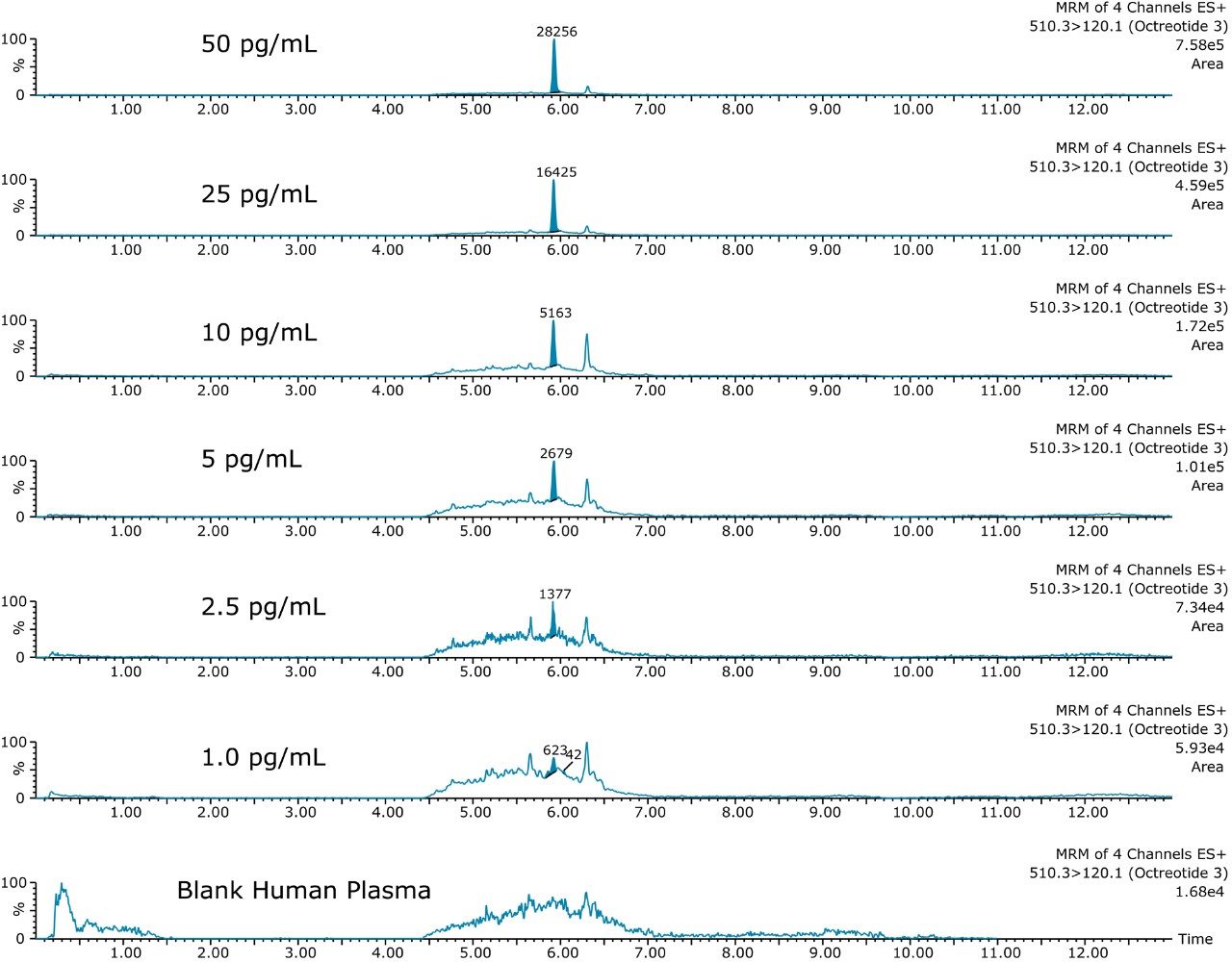
The combination of the ionKey/MS System and mixed-mode µElution SPE provided enhanced selectivity and increased sensitivity, whilst simultaneously significantly reducing sample volume requirements. Use of µElution format SPE eliminated the need for evaporation, reducing peptide losses due to adsorption and non-specific binding. The 150 µm iKey Separation Device enabled the development of a highly sensitive, low flow quantitative MRM method that simultaneously detects vasopressin, desmopressin, and octreotide with LOD <1pg/mL, and dynamic ranges from 1–2,000 pg/mL. The current analysis uses 100 µL of plasma and provides a significant improvement in sensitivity and S:N over analytical scale (2.1 mm I.D.) analysis. Furthermore, detection limits of 2.5 pg/mL are achievable from only 25 µL of plasma. In addition, the ionKey/MS System reduces solvent and sample consumption, thereby reducing cost and allowing for multiple injections of samples for improved accuracy or to meet the guidelines for incurred sample reanalysis (ISR).
720005128, January 2016