
In this application, the USP method for the impurity analysis of loratidine was transferred between three different columns. CORTECS Columns are ideal for updating the original method from 5 μm particle sizes to both 2.7 μm and 1.6 μm particle sizes. With the addition of the new CORTECS C8 chemistry, USP monographs that require L7 columns can be modernized to these highly efficient columns. By taking advantage of the newest column and separation technology such as CORTECS Columns, an analyst can achieve faster analysis times using less solvent, without sacrificing column efficiency. By using CORTECS C8 Columns the analysis of loratidine and related compounds was performed up to ten times faster, while consuming ten times less solvent, resulting in both a time and cost per analysis savings.
The United States Pharmacopeia (USP) has standards, enforced by government regulators, for how formulated pharmaceutical compounds should be analyzed. USP methods often use large columns, typically 4.6 mm I.D., packed with >3 μm particles which result in long analytical run times and consume large quantities of mobile phase.
One way to reduce the analytical run time, thereby decreasing turnaround time and solvent consumption, is to transfer the original USP method to a smaller column, packed with highly efficient solid-core particles1; CORTECS Columns are ideal for such modernization initiatives. CORTECS Columns contain solidcore particles with various bonded ligand groups. The newest additions to the CORTECS family are the Phenyl and C8 chemistries. These new chemistries allow USP monographs requiring L11 and L7 columns, respectively, to be modernized which provides faster analytical run times without sacrificing efficiency.
In this application, the USP method for the impurity analysis of loratidine was transferred between three different columns. The method transfer spanned three different particle sizes and used USP General Chapter <621> as a guideline.
|
LC conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC H-Class System |
|
Columns: |
XBridge BEH C8, 5 μm, 4.6 x 150 mm CORTECS C8, 2.7 μm, 3 x 100 mm CORTECS UPLC C8, 1.6 μm, 2.1 x 50 mm |
|
Mobile phase: |
Acetonitrile:methanol:0.01 M dibasic potassium phosphate (6:6:7) adjusted with phosphoric acid to an apparent pH of 7.2 |
|
Separation technique: |
Isocratic |
|
Flow rate: |
1.0 mL/min (5 μm column) 0.8 mL/min (2.7 μm column) 0.6 mL/min (1.6 μm column) |
|
Column temp.: |
30 °C |
|
Detection (UV): |
254 nm |
|
Injection volume: |
15.0 μL (5 μm) 4.3 μL (2.7 μm) 1.0 μL (1.6 μm) |
|
Data management software: |
Empower 3 CDS |
A sample containing loratidine (40 μg/mL), loratidine related compound A (10 μg/mL), and loratidine related compound B (10 μg/mL) was created using the 260:260:400:80 acetonitrile:methanol:0.05 N HCl:0.6M dibasic potassium phosphate.
Loratidine is a pharmaceutical compound used to help treat allergies. It is available over the counter under several trade names, including Claritin. Loratidine, shown in Figure 1, has two related compounds required for USP analysis. All three compounds contain the same four ring structure with some slight differences in bonding.
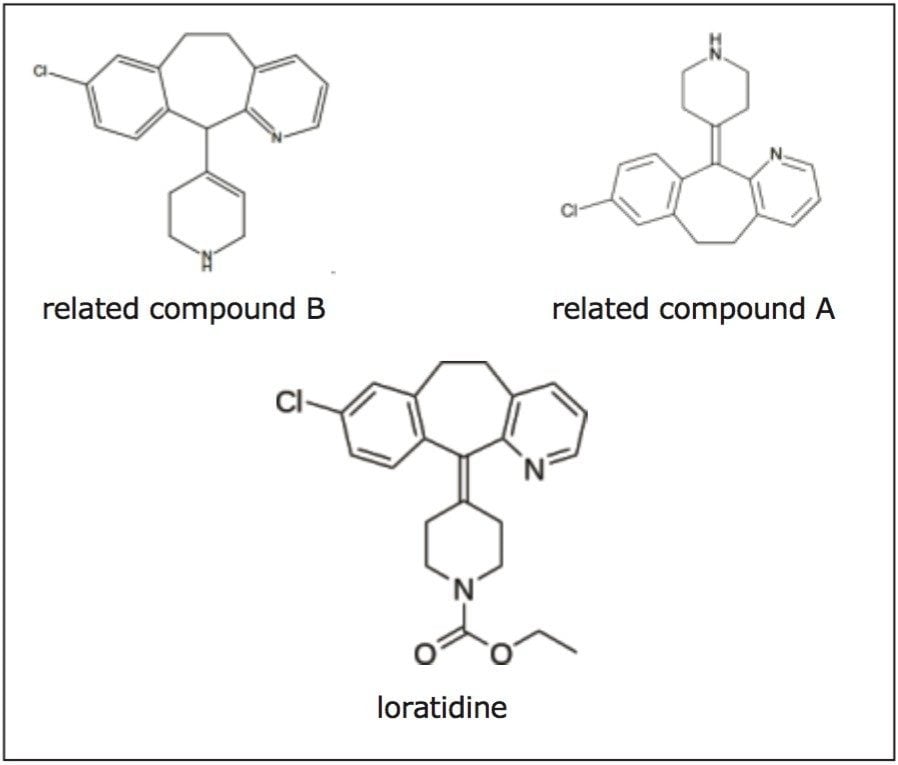
The original USP monograph for the analysis of loratidine impurities is performed on a 4.6 mm x 150 mm, 5 μm L7 column, which is a column with a C8 ligand. To be consistent with the USP monograph an XBridge BEH C8 Column, 5 μm, 4.6 x 150 mm (p/n 186003017), was used to separate the analytes on an ACQUITY UPLC H-Class System. Figure 2 shows the chromatogram of the separation on the XBridge BEH Column.
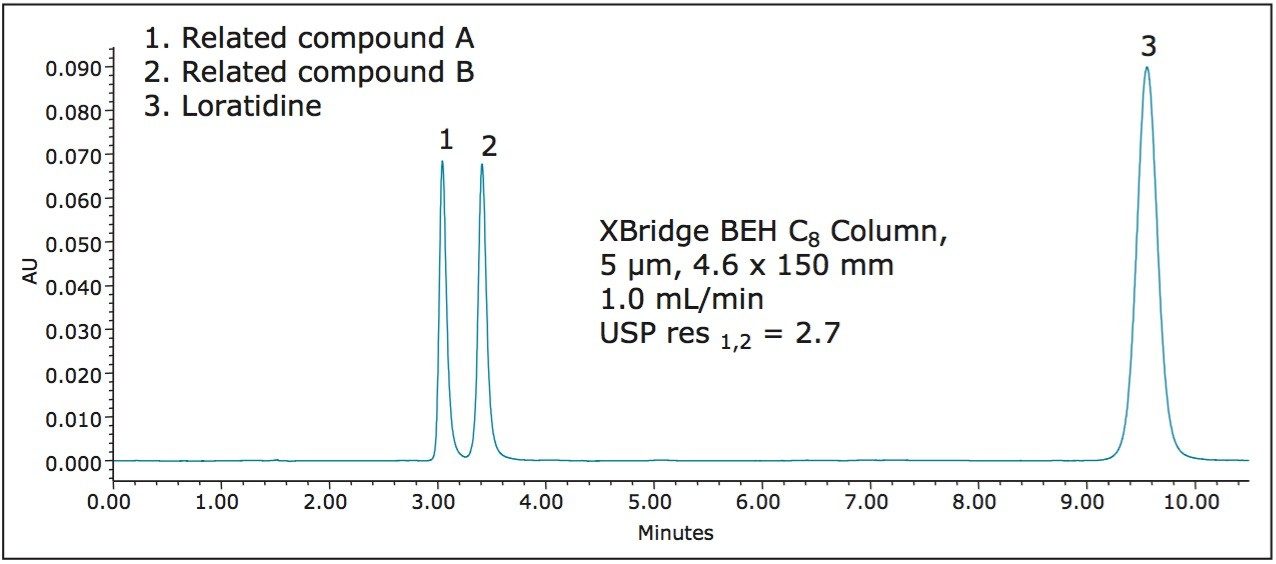
As outlined in the monograph, the system suitability criterion consists of two parts. The USP resolution between related compound A and B must be not less than (NLT) 1.5 and the percent RSD for retention time of loratidine can be not more than (NMT) 2.0%. For this separation, the resolution between related compounds A and B was 2.7, well within the requirements of the monograph. Triplicate injections were performed and the percent RSD for loratidine retention time was 0.74%. The results from the update of the monograph are found in Table 1.
In the last peak, loratidine elutes at approximately 9.6 minutes, providing room for improvement. At a flow rate of 1.0 mL/min, every injection with this column and method requires approximately 11 mL of solvent. By utilizing CORTECS Columns to update the separation, analysts can save time and money.
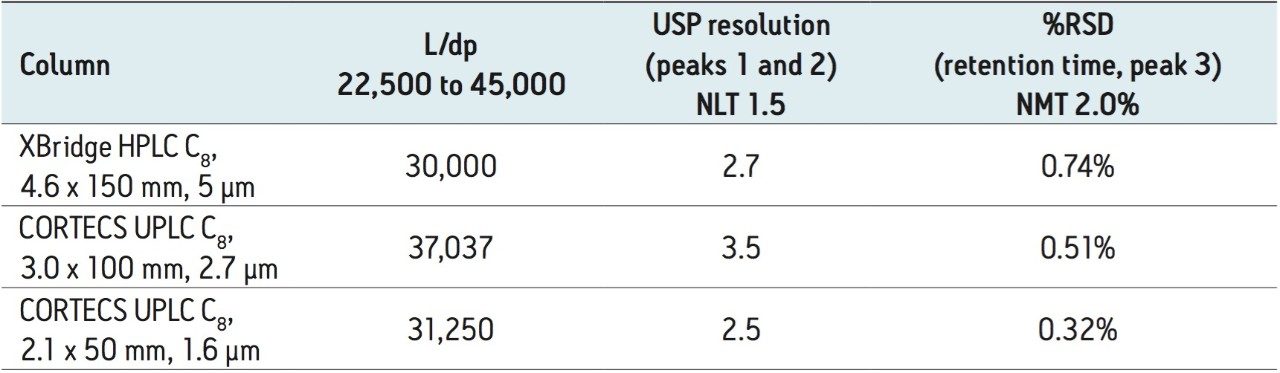
The USP guidelines allow specific changes to a monograph. Under USP General Chapter <621> Chromatography, for isocratic separations, the column configuration can be changed while maintaining the L/dp ratio (length to particle size ratio) within -25% and +50% of the original column used in the monograph. The L/dp ratio is a measure of a column’s resolving power. As shown in Table 1, the original column has an L/dp ratio of 30,000. In order to transfer this monograph the new column configurations must have L/dp ratios between 22,500 and 45,000.
For the analysis of loratidine, the new CORTECS C8 Columns were used. CORTECS 2.7 μm Columns are designed to perform separations on HPLC and UHPLC instrumentation while operating within the pressure limits of the system. In order to use a 2.7 μm particle column, a length of 100 mm can be used because, as Table 1 shows, the L/dp ratio of such a column is 37,037, which is within the range specified above. After scaling the flow rate and injection volume using the ACQUITY UPLC Columns Calculator, the separation was performed, and the chromatogram is shown in Figure 3.
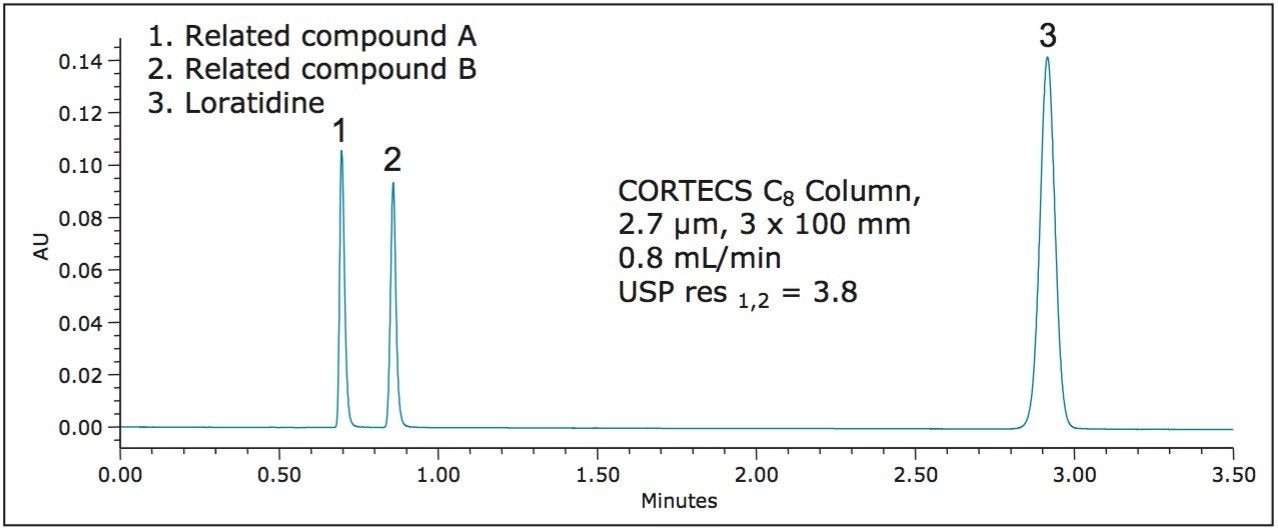
Here, loratidine elutes at approximately 2.9 minutes, an approximate threefold decrease vs. the USP method above, while using approximately four times less solvent. As Table 1 shows, all of the system suitability requirements are met for this separation.
CORTECS Columns are also available packed with 1.6 μm particles. These 1.6 μm columns are designed for use on UPLC Systems. Whether analyzing a complex mixture or increasing throughput without sacrificing efficiency, the CORTECS 1.6 μm Columns are ideal for analysts who want the highest efficiency possible. In order to be compliant with the above USP General Chapter <621> L/dp guideline, a 50 mm column geometry was chosen. The L/dp ratio for this particular column configuration is 31,250, which is within the acceptable limits. Figure 4 shows the separation of loratidine and related compounds on a CORTECS UPLC C8 Column, 1.6 μm, 2.1 x 50 mm.
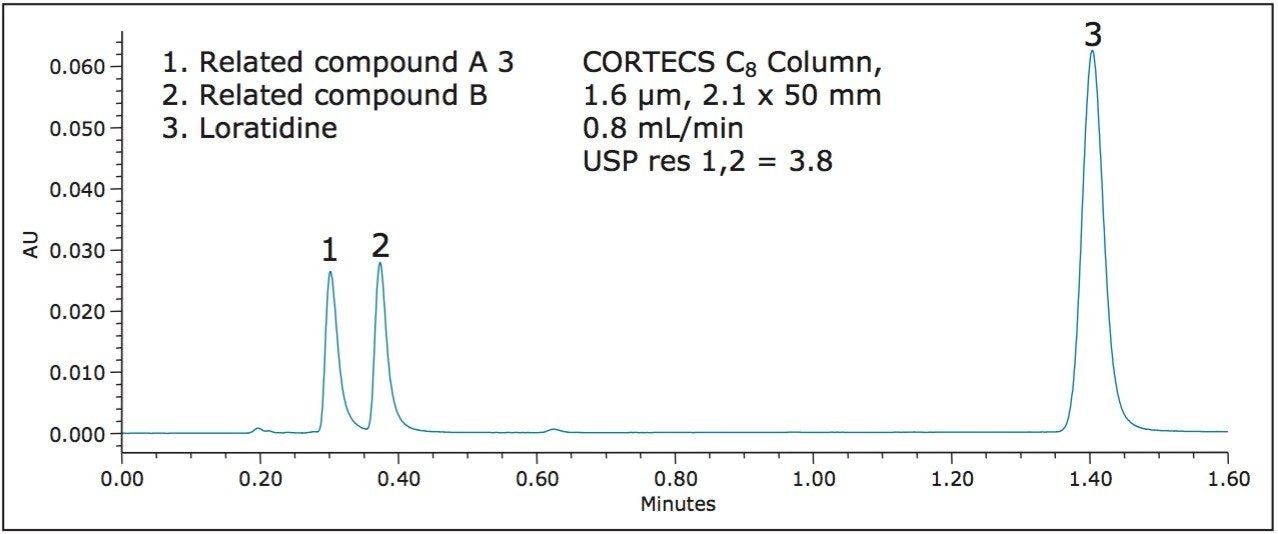
With the newest column technologies, the loratidine peak appears at approximately 1.40 minutes. Compared to the compendial USP method, this is approximately a tenfold decrease in sample run time, while using 10 times less solvent.
The separation of loratidine and related compounds was transferred from a 5 μm HPLC column to CORTECS 2.7 μm and 1.6 μm Columns, demonstrating a successful update of a USP monograph.
USP methods often implement older column and separation technology, requiring lengthy analysis run time and large quantities of mobile phase. However, USP guidelines specify that methods can be altered as outlined in USP General Chapter <621>. CORTECS Columns are ideal for updating the original method from 5 μm particle sizes to both 2.7 μm and 1.6 μm particle sizes. With the addition of the new CORTECS C8 chemistry, USP monographs that require L7 columns can be modernized to these highly efficient columns. By taking advantage of the newest column and separation technology such as CORTECS Columns, an analyst can achieve faster analysis times using less solvent, without sacrificing column efficiency. By using CORTECS C8 Columns the analysis of loratidine and related compounds was performed up to ten times faster, while consuming ten times less solvent, resulting in both a time and cost per analysis savings.
720005579, January 2016