This application note shows the analysis of eleven bisphenol compounds, including BPA, using a CORTECS UPLC Phenyl Column on an ACQUITY UPLC H-Class System.
The separation of eleven bisphenol compounds was achieved by using the CORTECS UPLC Phenyl Column with an ACQUITY UPLC H-Class System. By using this technology, baseline separation was achieved for each compound with a ten-minute-gradient run. The developed bisphenol screening method allows for the analysis of extracted bisphenols from consumer products such as receipts. Understanding how bisphenol compounds behave in the environment is critical to their use and regulation, and CORTECS UPLC Phenyl Columns are a useful tool in analyzing these hazardous compounds.
Bisphenol A (BPA) is a chemical compound used in the processing of plastics and resins, as well as receipt paper. Recently, the use of BPA has been reduced due to the negative effects it has on both the environment and consumers. BPA is a known endocrine disruptor mimicking estradiol when introduced into a human’s metabolic cycle.1 The disruption of the endocrine system can lead to hormone imbalances, and estrogen disruptors, such as BPA, have been linked to infertility.2 Consumers regularly come into contact with products containing BPA or one of the many other bisphenol compounds.
This application note shows the analysis of eleven bisphenol compounds, including BPA, using a CORTECS UPLC Phenyl Column on an ACQUITY UPLC H-Class System. Due to the concern over negative effects of BPA, manufacturers are beginning to replace it with other bisphenol compounds, such as Bisphenol S (BPS). However, recent studies indicate that BPS also displays signs of disrupting cell signaling and may not be a safe alternative.3 By using a simple screening technique, an analyst can determine if any of the bisphenols are present in their sample. After developing a baseline separation for all eleven compounds, readily available receipt papers were analyzed for BPA or any of the other bisphenols.
|
Columns: |
CORTECS UPLC Phenyl, 1.6 μm, 2.1 x 100 mm (p/n: 186008381) |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Methanol |
|
Mobile phase D: |
2% formic acid in water (autoblended to 0.1% formic acid) |
|
Gradient: |
See Table 1 |
|
Flow rate: |
0.3 mL/min |
|
Column temp.: |
30 °C |
|
Detection (UV): |
275 nm |
|
Injection volume: |
0.8 μL |
|
Data management: |
MassLynx 4.1 |
|
Time |
%A |
%B |
Curve |
|---|---|---|---|
|
0.00 |
45 |
50 |
5 |
|
8.39 |
0 |
95 |
5 |
|
10.00 |
0 |
95 |
5 |
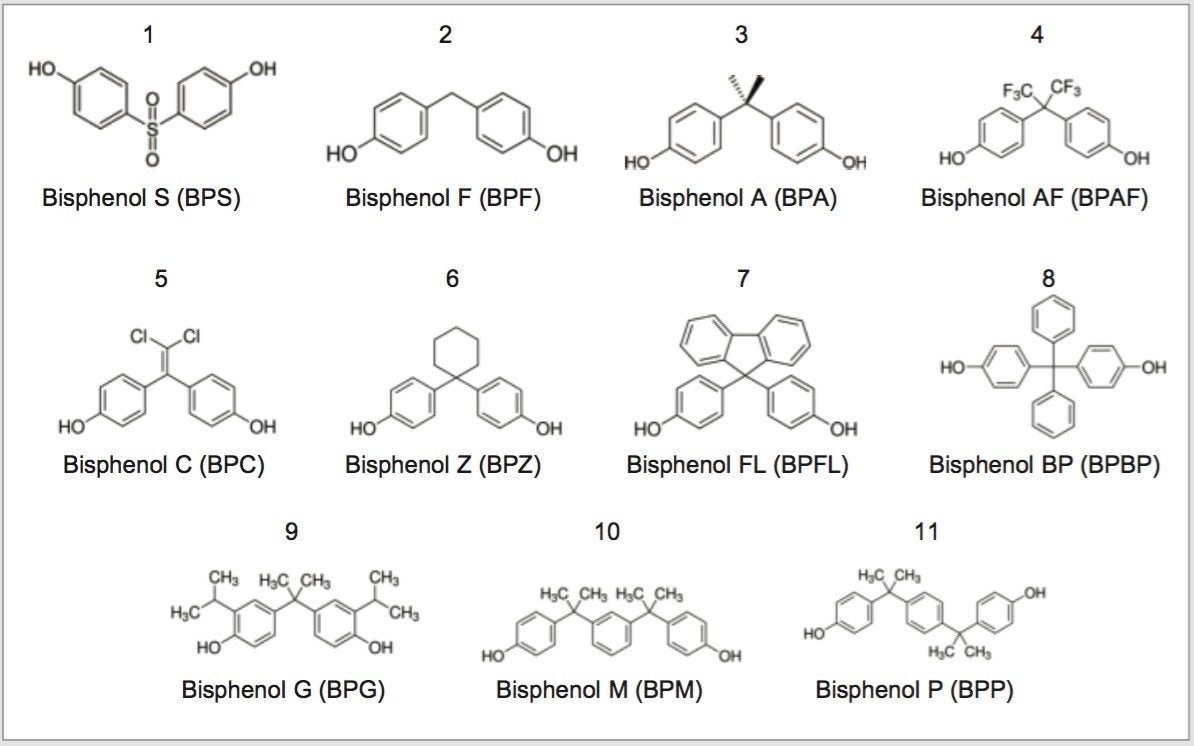
Stock solutions (1 mg/mL) of each compound shown in Figure 1 were created. Aliquots of these solutions were combined in a single vial and diluted to a final concentration of 0.1 mg/mL, except for bisphenol S (0.75 mg/mL) using 50:50 water:methanol as the diluent.
Four different receipts were tested. Each receipt was trimmed to approximately equal size and each resulting piece was cut in half. The first half of each receipt was subjected to extraction whereby each sample was incubated with 20 mL methanol for 3 hours at room temperature. The solutions were filtered, and an aliquot of each was taken for analysis. The masses of the receipt halves were:
Receipts:
Yellow carbonless copy receipt – 0.28 g
Gas station receipt – 0.20 g
Hardware store receipt – 0.24 g
Commuter rail receipt – 0.18 g
The second half of each receipt was subjected to contact transfer by swiping a slightly damp (with water) Kimwipe across the face of each receipt piece. Each Kimwipe was incubated with 20 mL methanol for 3 hours at room temperature. The solutions were filtered, and an aliquot of each was taken for analysis.
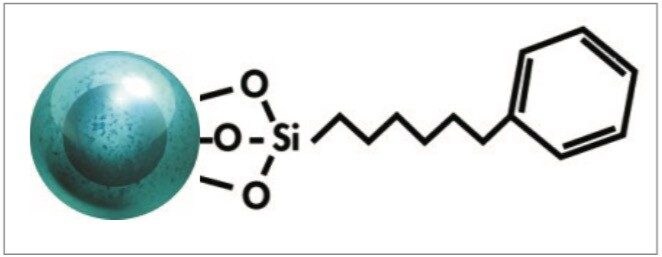
CORTECS UPLC Phenyl Columns contain a phenyl ligand attached to a solid-core particle, as shown in Figure 2. The phenyl ligand on the particle produces a unique selectivity compared to traditional carbon chain ligands, such as a C18 or C8.4 The pi bonds in the ligand may interact with any pi electrons in an analyte, giving a secondary separation mechanism in addition to hydrophobicity.
As seen in Figure 1, all of the bisphenol compounds have similar structures. The two phenol groups, attached to a central atom or group, offer many pi electrons that can interact with the phenyl stationary phase. In order to take advantage of this interaction, methanol was used as the strong solvent for the gradient method development. Methanol augments the pi-pi interaction of the analyte with the stationary phase whereas a solvent like acetonitrile reduces that interaction.4,5 For this particular mixture of analytes, utilizing the pi-pi interactions increases the chances of having a successful separation.
Using a gradient method, the eleven bisphenol compounds were successfully separated. The chromatogram of the separation is shown in Figure 3.

The baseline separation of the eleven bisphenol compounds was achieved in 8 minutes, with bisphenol P (BPP), 11, eluting at ~7.9 minutes. BPA, 3, is well separated from the other bisphenol compounds. While BPA is the main compound of interest, other bisphenol compounds are gaining importance in both manufacturing as well as environmental testing. Bisphenol S (BPS), 1, bisphenol F (BPF), 2, and bisphenol AF (BPAF), 4, for instance are being used as potential replacements for BPA in certain products.6 Developing a separation method for these eleven compounds proves beneficial for the analysis of real world samples, both for identification and the potential quantitation of any compounds found.
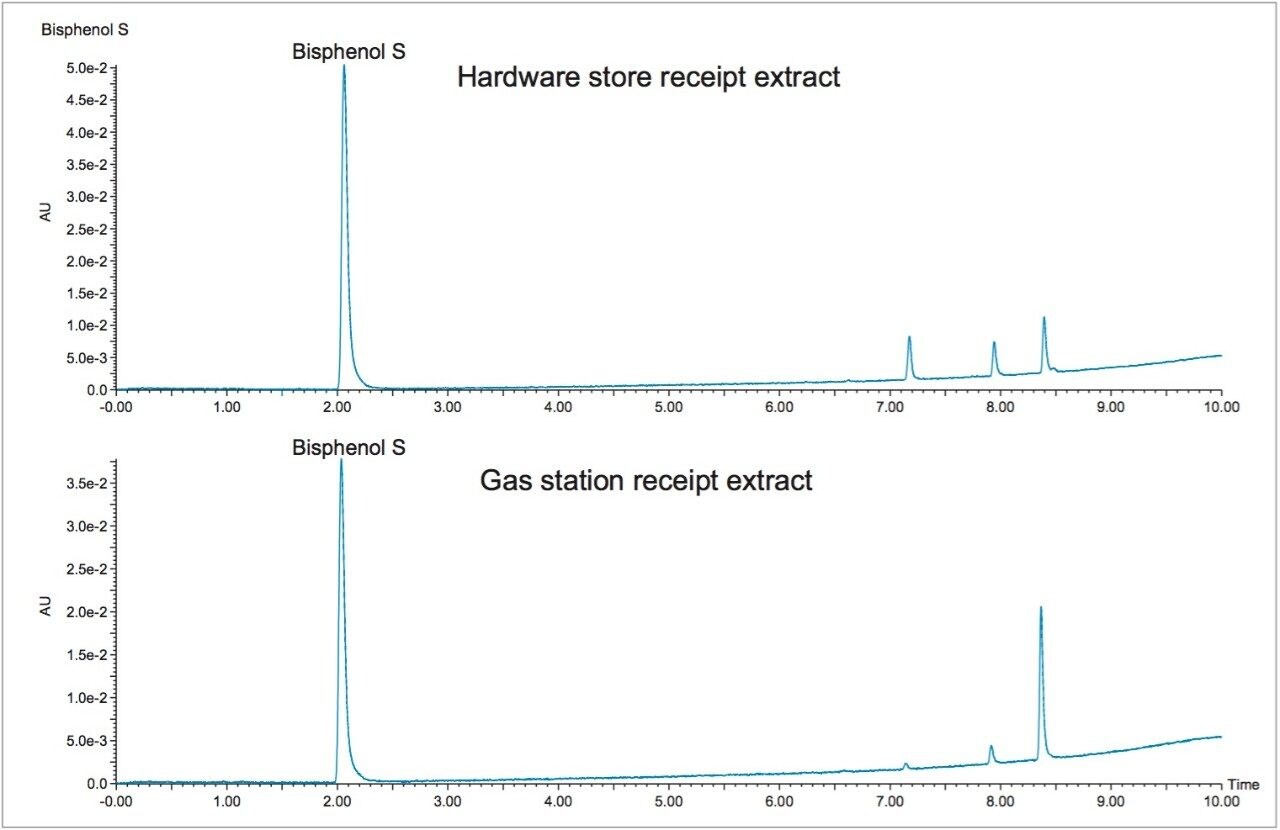
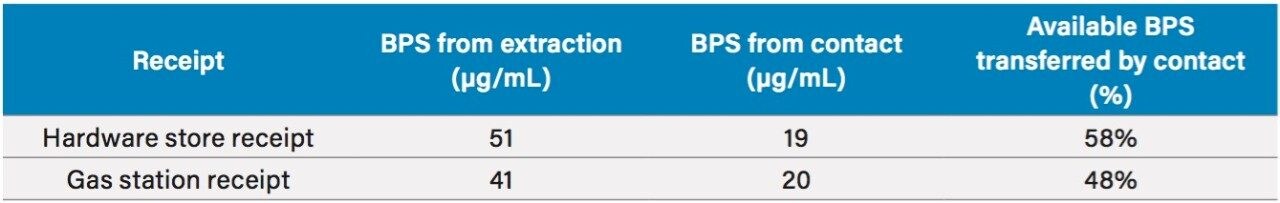
The developed bisphenol screening method was used for the analysis of paper receipts. A yellow carbonless paper copy and receipts from a train ticket, hardware store, and gas station were analyzed for bisphenols. Two of these, the yellow carbonless paper copy and the commuter rail receipt, showed no signs of bisphenols after sample extraction and analysis by UPLC with UV detection. BPS was the only bisphenol compound extracted from the hardware store and gas station receipts.
The chromatograms of the extracts from these receipts can be seen in Figure 4, with BPS eluting at around 2 minutes and verified through mass spectrometry analysis. Mass spectrometry analysis also confirmed that the unidentified peaks were not any of the remaining ten bisphenol compounds.
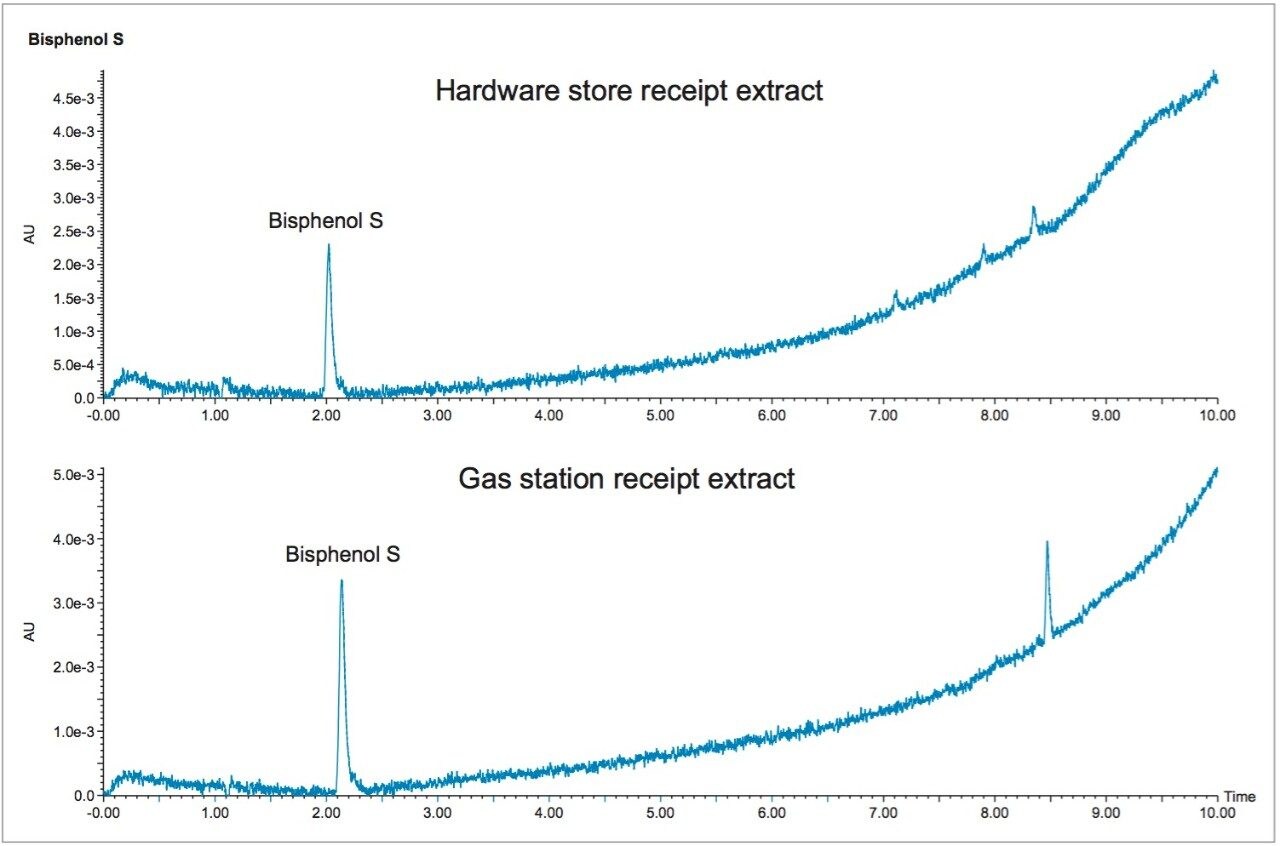
The receipts were also tested for signs of BPS transfer during handling. This was accomplished by wetting a Kimwipe with water and swiping it across the face of the receipt for sample analysis. The slightly damp Kimwipe represents the moisture from skin when touching the receipts and is a surrogate for BPS transfer to the skin through physical contact. The chromatograms in Figure 5 show the results of this test. BPS is seen in each sample, indicating that BPS may be transferred simply through touching receipts.
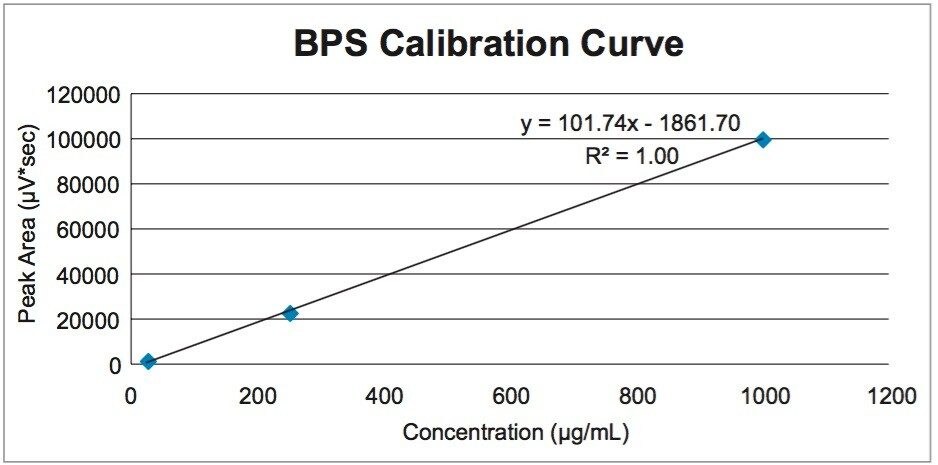
From these data, the extracted BPS from the sample receipts as well as the amount transferred during handling can be calculated. Table 2 gives the initial masses of each sample and the calculated amounts of extracted and transferred BPS. A simple calibration curve was created to calculate the amount of BPS in each sample, as shown in Figure 6. The three point calibration curve was created to cover a range of 25–1000 µg/mL.
The separation of eleven bisphenol compounds was achieved by using the CORTECS UPLC Phenyl Column with an ACQUITY UPLC H-Class System. By using this technology, baseline separation was achieved for each compound with a ten-minute-gradient run. The developed bisphenol screening method allows for the analysis of extracted bisphenols from consumer products such as receipts. Understanding how bisphenol compounds behave in the environment is critical to their use and regulation, and CORTECS UPLC Phenyl Columns are a useful tool in analyzing these hazardous compounds.
720005900, February 2017