
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates development of faster methods with lower solvent consumption using CORTECS columns in USP method modernizations.
Faster methods with lower solvent consumption using CORTECS C8 and CORTECS UPLC C8 Columns in USP method modernizations.
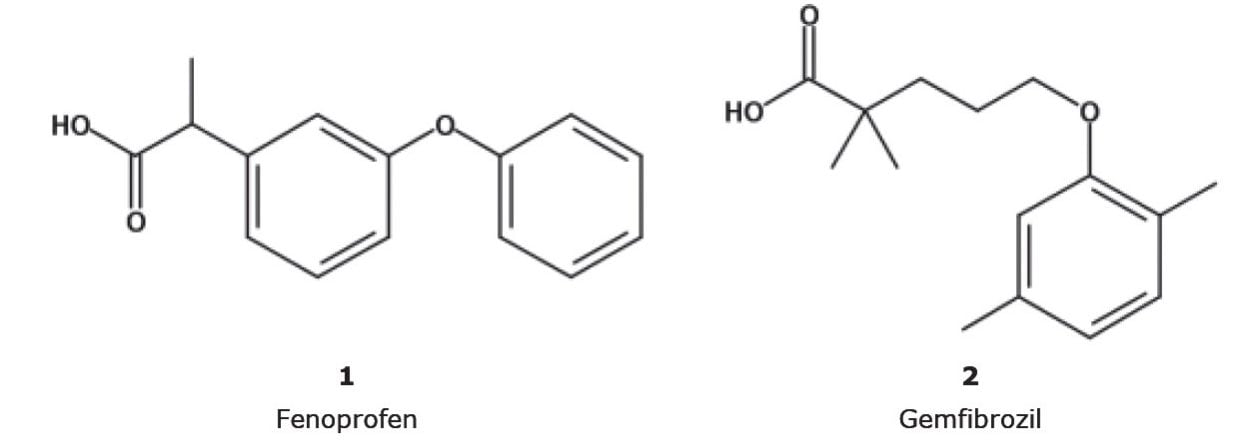
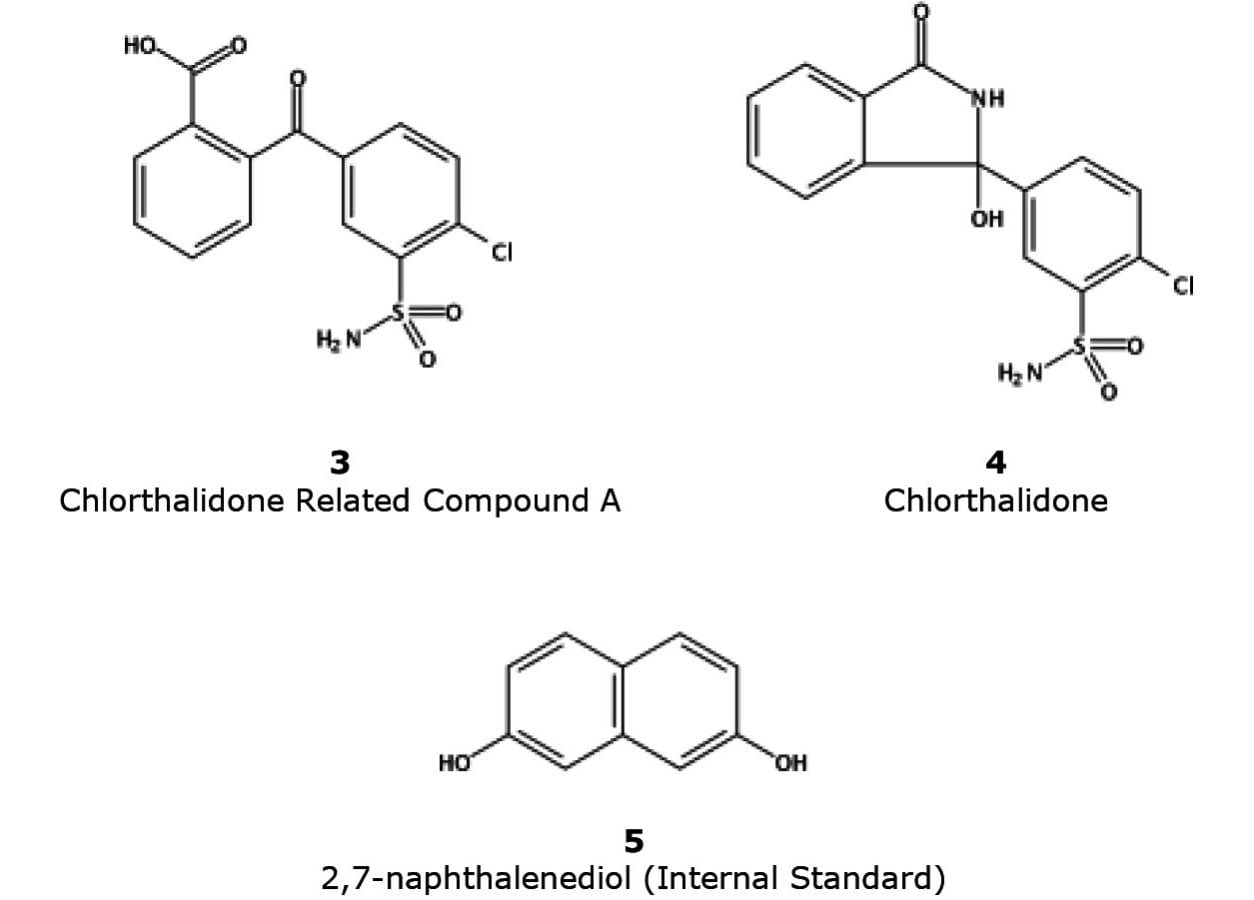
Fenoprofen is an anti-inflammatory drug used to treat pain and arthritis. The resolution standard in the USP monograph for fenoprofen¹ separates fenoprofen and gemfibrozil. Chlorthalidone is a nonthiazide diuretic used to prevent heart failure by lowering high blood pressure and edema. Its USP monograph describes a method to separate chlorthalidone, its related impurity, and an internal standard.² Many of these USP methods are considered “outdated” due to the use of old column technologies, which can leave analysts with long sample run times coupled with high solvent consumption.
Since August 2014, USP method modernizations for isocratic separations may now occur by following “Equivalent L/dp” or “Equivalent N” guidelines in relation to the original column.³ For “Equivalent L/dp”, the ratio of column length, L, to the particle size (diameter), dp, must be maintained within -25% to +50% of the original L/dp, and for “Equivalent N”, other combinations of L/dp may be used as long as N remains within -25% to +50% of that measured in the original method. High efficiency columns packed with <3 µm solid-core particles, as found in the CORTECS (2.7 µm) and the CORTECS UPLC (1.6 µm) families, allow shorter columns to be used while maintaining equivalent performance to the original USP method.⁴ This results in faster methods with less solvent consumption.
It is important to match the chromatographic instrument dispersion and pressure limit with the column dispersion and backpressure.
However, using a column with a more capable instrument (e.g. a 3.0 mm diameter, 2.7 μm particle column on a UPLC) is feasible and often convenient when the optimal dimension is unavailable. This study therefore demonstrates this practicability by using 3.0 mm diameter columns packed with 2.7 μm and 1.6 μm CORTECS particles on an ACQUITY UPLC H-Class System.
Figure 3 shows the analysis of fenoprofen and gemfibrozil on CORTECS and CORTECS UPLC C8 Columns that meet either “Equivalent L/dp” or “Equivalent N” allowed changes. An ACQUITY UPLC H-Class System with Auto•Blend was used with a mobile phase composition of 50:49.6:0.4 acetonitrile:water:phosphoric acid and UV detection at 272 nm. The original column – a ZORBAX C8, 5 µm, 4.6 x 150 mm – was run at a flow rate of 2.00 mL/min on an Alliance HPLC System. The flow rate for the 2.7 µm columns was scaled to 1.58 mL/min, and the flow rate for the 1.6 µm columns was set to 1.40 mL/min due to system pressure limits.
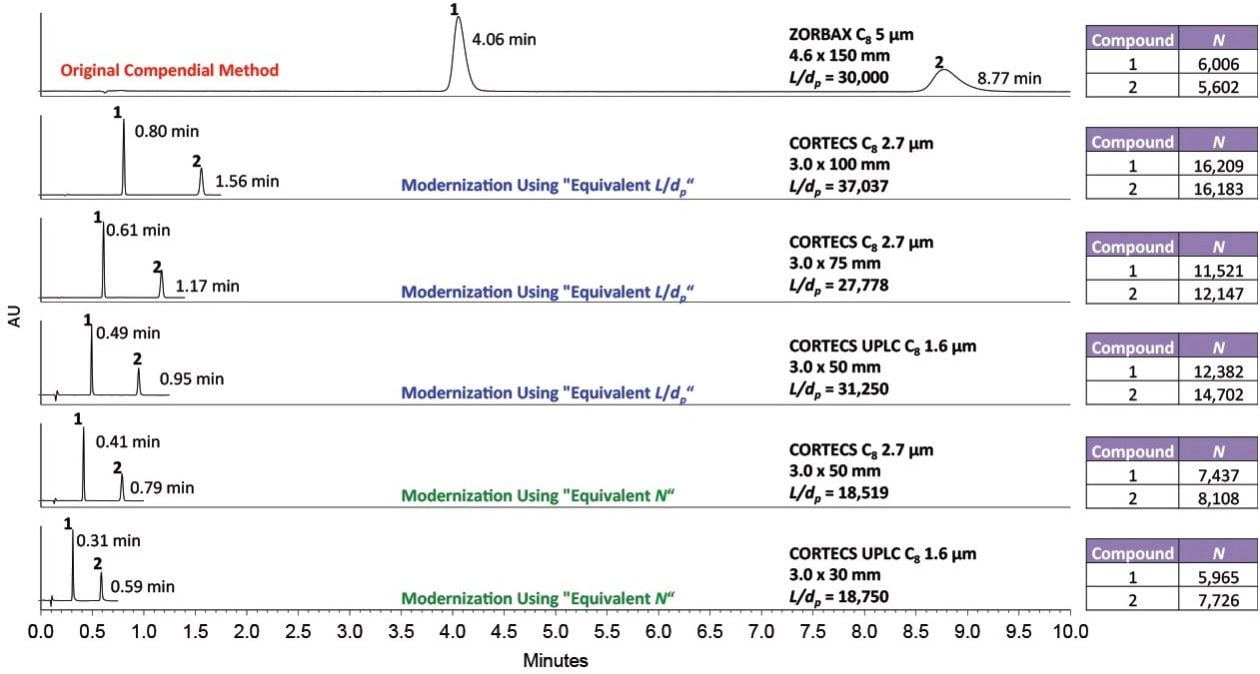
All columns met the USP specifications outlined in the monograph. A decrease in analysis time of 93% occurred when switching to a column following the “Equivalent N” criteria – a CORTECS UPLC C8, 1.6 µm, 3.0 x 30 mm Column (p/n: 186008408). Using this column, solvent consumption also decreased by 95%.
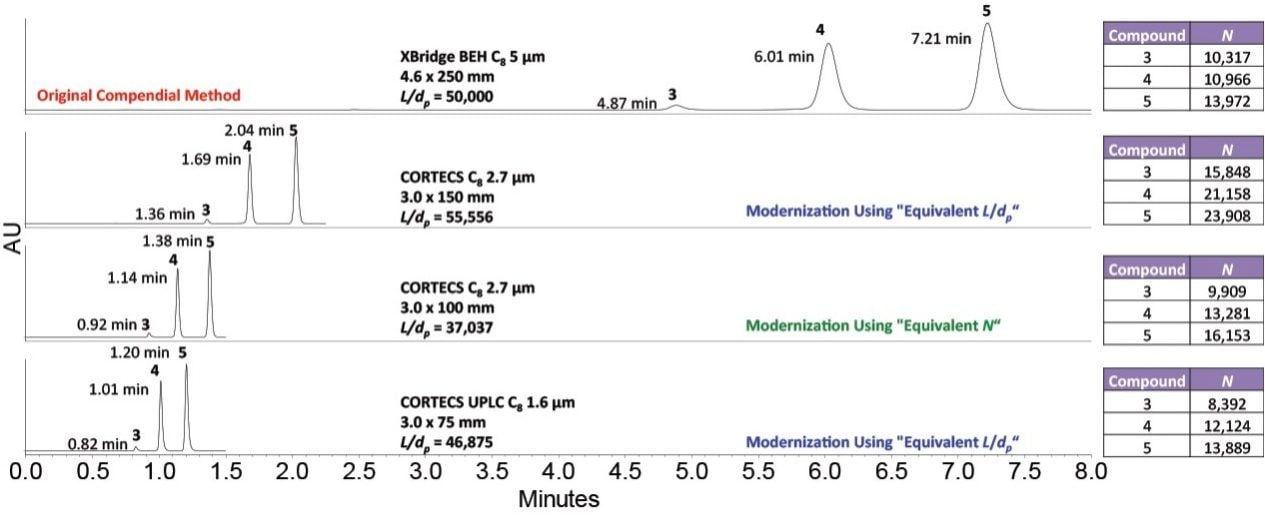
Figure 4 depicts the modernization of the USP method for chlorthalidone using CORTECS C8 and CORTECS UPLC C8 Columns. The original compendial column – XBridge BEH C8, 5 µm, 4.6 x 250 mm (p/n: 186003018) – was run on an Alliance HPLC System using a mobile phase of 0.01 M dibasic ammonium phosphate:methanol (3:2) adjusted with phosphoric acid to pH 5.5. The analysis used a flow rate of 1.00 mL/min with UV detection at 254 nm. The modernized methods using CORTECS Columns were run on an ACQUITY UPLC H-Class System at a scaled flow rate of 0.79 mL/min for 2.7 µm columns and, in order to maintain operable system pressures, 0.70 mL/min for the 1.6 µm column.
The columns in Figure 4 all met the required USP specifications. However, while the USP modernization for fenoprofen used the “Equivalent N” guideline to give the largest reduction in analysis time and solvent consumption, the “Equivalent L/dp” guideline provided the greatest advantage for chlorthalidone. The sample run time can be cut six fold using a CORTECS UPLC C8, 3.0 x 75 mm Column (p/n: 186008410), and solvent consumption can be reduced by up to 88%.
The USP monograph methods for fenoprofen and chlorthalidone were successfully transferred from fully-porous C8 columns to solid-core CORTECS C8 and CORTECS UPLC C8 Columns. By switching to solid-core particles while utilizing the “Equivalent L/dp” and “Equivalent N” allowed changes for method adjustments, USP methods can be modernized for higher sample throughput while maintaining and even increasing chromatographic performance. The successful method modernizations of fenoprofen and chlorthalidone demonstrate the ability of CORTECS Columns to drastically decrease sample analysis time and reduce solvent consumption using both “Equivalent L/dp” and “Equivalent N” guidelines.
720005988, April 2017