For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application note demonstrates the use of the Kairos Amino Acid Kit with the MetaboQuan-R LC-MS/MS platform for the quantitative, high-throughput profiling of amino acids.
Amino acids are extremely important biomolecules and are relevant in many branches of biomedical research. Here, we demonstrate the use of the Kairos Amino Acid Kit with the high-throughput, targeted, multi-omics LC-MS/MS platform, MetaboQuan-R. By doing so, we are able to produce a rapid workflow that delivers reproducible, quantitative, amino acid analysis.
Amino acids are the constituent building blocks of proteins and are, therefore, central to all aspects of biological function. The accurate, precise, and reproducible measurement of amino acids is critical in many branches of biomedical research. The analysis of these compounds is typically performed using derivatization followed by flow injection analysis (FIA) tandem mass spectrometry (MS/MS) or FIA-MS/MS. A major limitation of FIA-MS/MS methods for amino acid analysis is the inability to distinguish isobaric species, leading to degraded accuracy and precision. Another limitation of many available amino acid analyses is the lack of standardized calibration standards and quality control samples, key components for reproducible studies. The use of quality-controlled consumables kit and stable-isotope labelled (SIL) standards, combined with a reproducible, high-throughput UPLC-MS/MS platform, provides a solution to these two issues.
Human serum samples were prepared as per the instructions provided in the Kairos Amino Acid Kit. In summary, samples were subjected to protein precipitation using sulfosalicylic acid that contained SIL internal standards. Samples were then derivatized using the AccQTag reagent before dilution and injection into the MetaboQuan-R LC-MS/MS workflow.
UPLC separation was performed with an ACQUITY UPLC I-Class System (Fixed Loop), equipped with a CORTECS T3 2.7 µm (2.1 x 30 mm) analytical column. A sample of 2 µL was injected at a flow rate of 1.3 mL/min. Mobile phase A was 0.01% formic acid (aq) containing 0.2 µM ammonium formate and mobile phase B was 50% isopropanol in acetonitrile containing 0.01% formic acid and 0.2 µM ammonium formate. The derivatized amino acids were eluted from the column and separated with a gradient of 1–8% mobile phase B over 2.4 minutes, followed by a 0.9 minute column wash at 98% mobile phase B. The column was then re-equilibrated to initial conditions. The analytical column temperature was maintained at 60°C.
Multiple Reaction Monitoring (MRM) analyses were performed using a Xevo TQ-S micro Mass Spectrometer. All experiments were performed in positive electrospray ionization (ESI+) mode. The ion source temperature and capillary voltage were kept constant at 150°C and 2.0 kV, respectively. The cone gas flow rate was 50 L/hr and desolvation temperature was 650 °C.
Calibration lines were created for the 29 amino acids detailed in Table 1 using the peak area ratio to a SIL internal standard. The SIL internal standard used for each analyte was either the heavy labelled analog of the analyte itself or the closest internal standard to the analyte in terms of chromatographic retention time. Figures 1–3 show examples of the calibration lines produced. These are for Glutamine, Homocitrulline, Lysine, and Tyrosine.
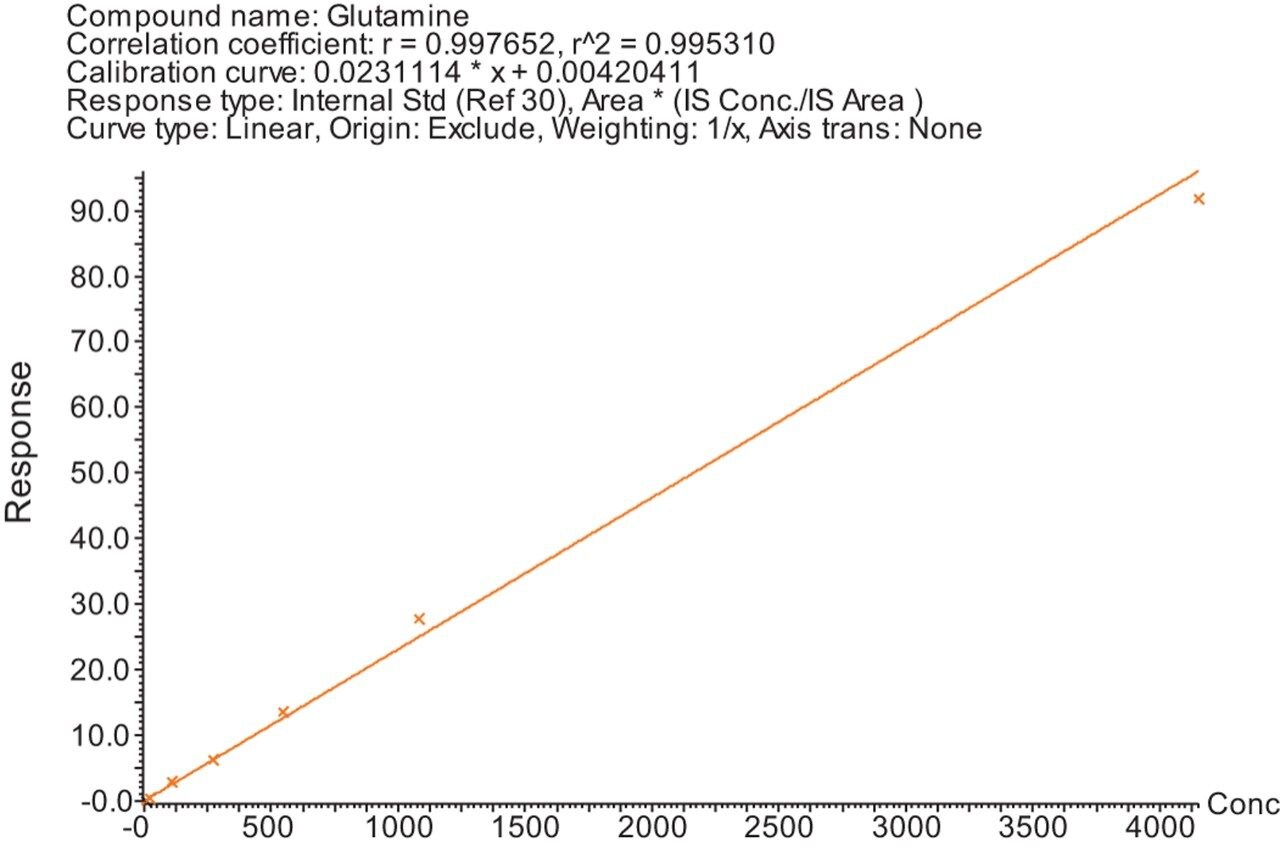
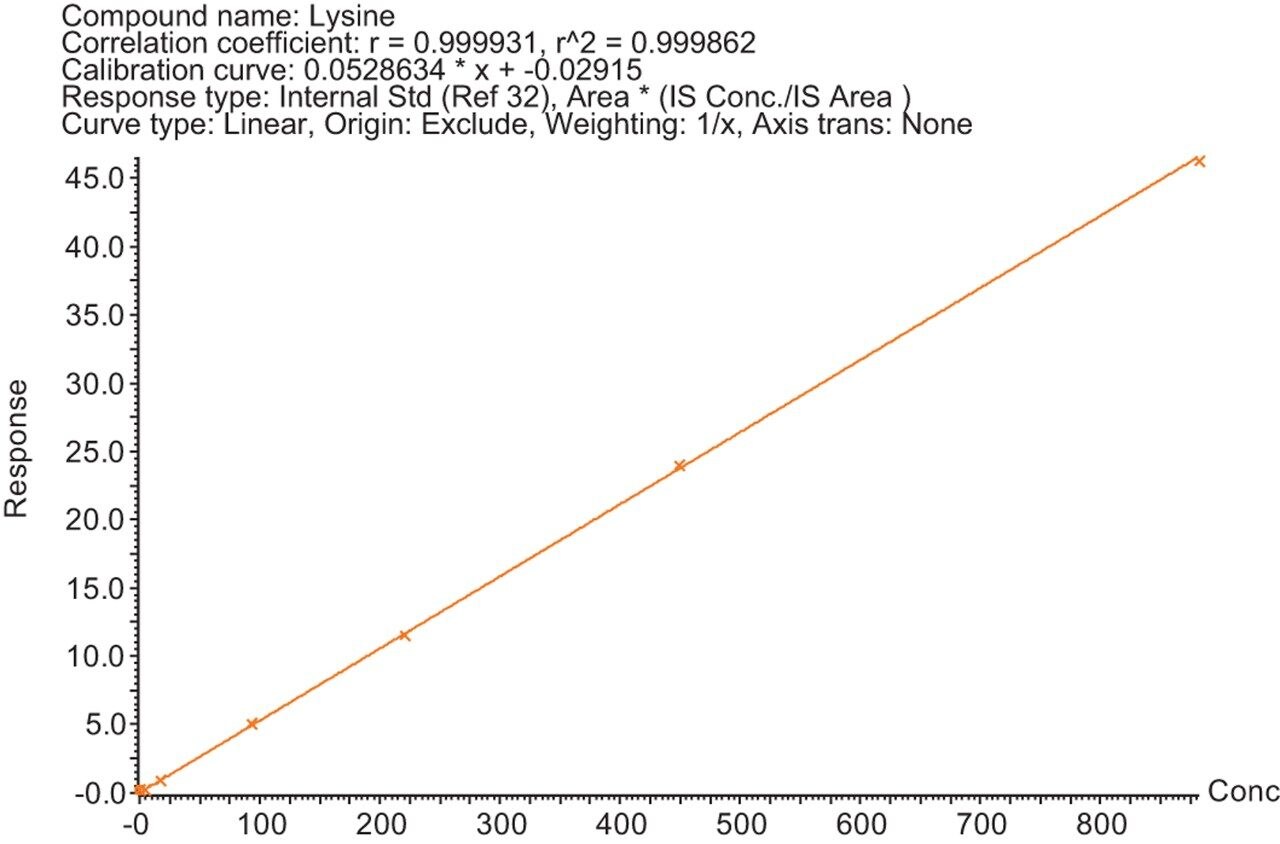
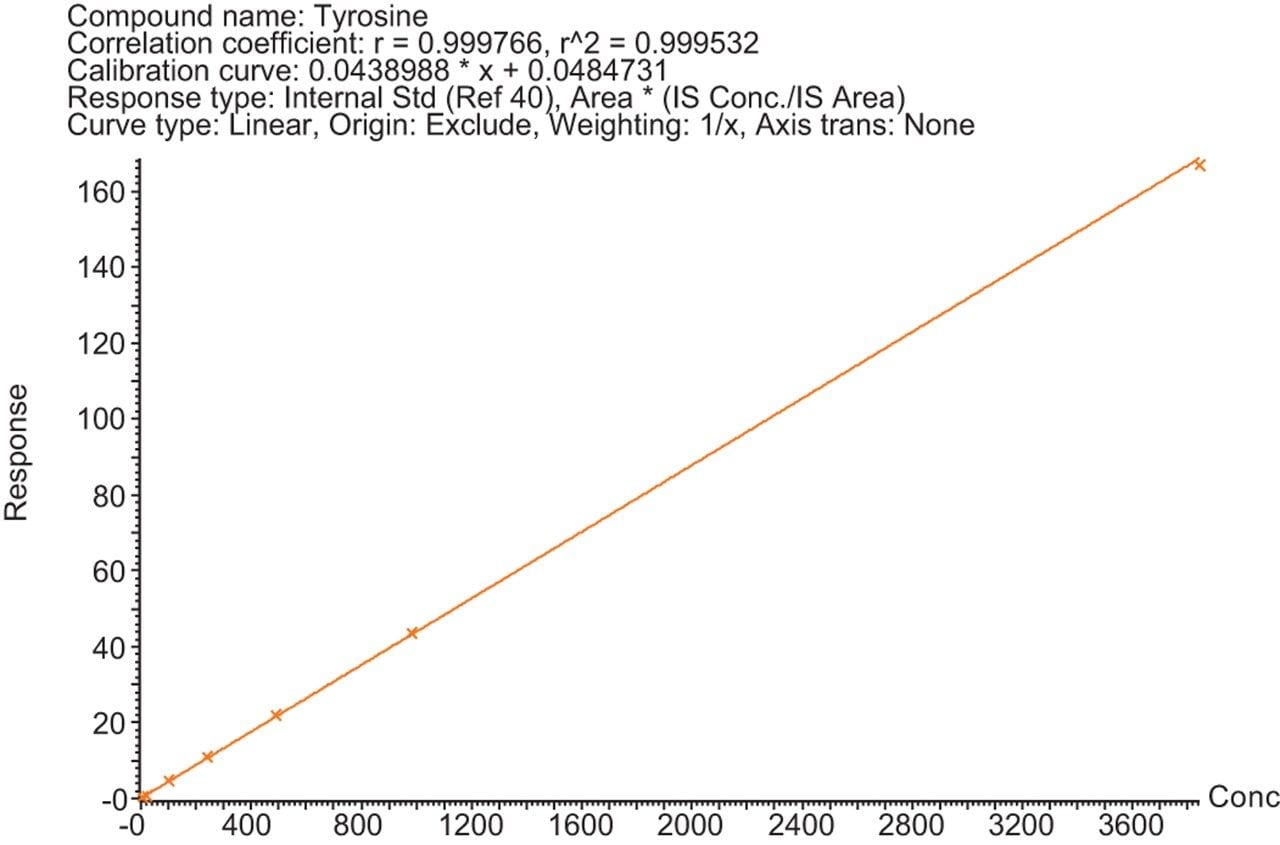
The Kairos Amino Acid Kit was successfully used on the MetaboQuan-R LC-MS/MS workflow for the quantitative analysis of amino acids. Excellent analytical specificity, linearity, and dynamic range were demonstrated. The combination of a standardized kit and LC-MS/MS workflow provides an optimized platform for the accurate, precise, and reproducible analysis of amino acids in biological samples and is ideally suited to a wide variety of biomedical research studies.
720006532, April 2019