This is an Application Brief and does not contain a detailed Experimental section.
For forensic toxicology use only.
This work demonstrates an automated simple dilute and shoot sample preparation method using the Andrew+ Pipetting Robot from Andrew Alliance. The Andrew+ developed method prepares the sample preparation of biomarkers ethylglucuronide (EtG) and ethylsulfate (EtS). At each protocol step during experiment execution, Andrew+ executes the OneLab instructions, setting the required parameters on the labware and Bluetooth pipettes to be used. OneLab is very accessible and straight forward to use for creating and transferring methods.
Sample identification and quantification is performed on an ACQUITY UPLC I-Class (FTN) System and Xevo TQD Mass Spectrometer using an ACQUITY UPLC CSH Phenyl-Hexyl Column (p/n: 186005408).
Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are important biomarkers for monitoring alcohol use. Identification and quantification of EtG and EtS as a biomarker of ethanol use is performed for a wide range of testing purposes. Detecting these metabolites has proven beneficial as alcohol abuse is highly prevalent in many cultures and contribute considerably to the global burden of health and social issues. As a result, there is a growing need for the detection and identification of ethanol use.
EtG and EtS are superior markers of alcohol intake due to a long detection window. EtG and EtS are minor water soluble II metabolites of ethanol and are detectable in urine up to 80 hours following ethanol consumption.1
The sample preparation method detailed here is based upon the “dilute and shoot” format. This method involves the dilution of samples with an internal standard before injection onto an LC-MS system.
The EtG/EtS “dilute and shoot” method on Andrew+ involves the development of accurate pipetting and mixing of samples on deck. This is a straightforward workflow to perform, however pipetting can still be a tedious and repetitive task especially when running larger sample numbers. The automation of this workflow frees up analyst expertise for other tasks and requires minimal training to run. The OneLab cloud native software guides the user when running a protocol with the required materials and deck setup. Andrew+ provides reproducible and consistent pipetting with every run, reducing the risk of user error that can be seen in manual sample preparation.
Two scripts were developed on the Andrew+ for an automated “dilute and shoot” sample preparation of EtG and EtS in human urine.
Two protocols were developed on the Andrew+ for a “dilute and shoot” automated method. Both protocol methods transferred a set of eight quality control low aliquots and eight quality control high aliquots to the first two rows of the 2 mL collection plate (p/n: 186002482) which were diluted with water (in place of internal standard). The difference in both automated methods was the mixing of the liquid in the wells. One automated method used pipette mixing and the other automated method used Microplate Shaker+ to perform mixing. The pipette liquid handling parameters were adjusted to ensure smooth transfer of samples. During development of protocol that performs pipette mixing of samples, adjustments were made to the pipette mixing parameters. An example of this was increasing the pipette mixing speed to ensure thorough mixing. Adjustments were straightforward to change in OneLab. This optimization helped increase reproducibility and precision.
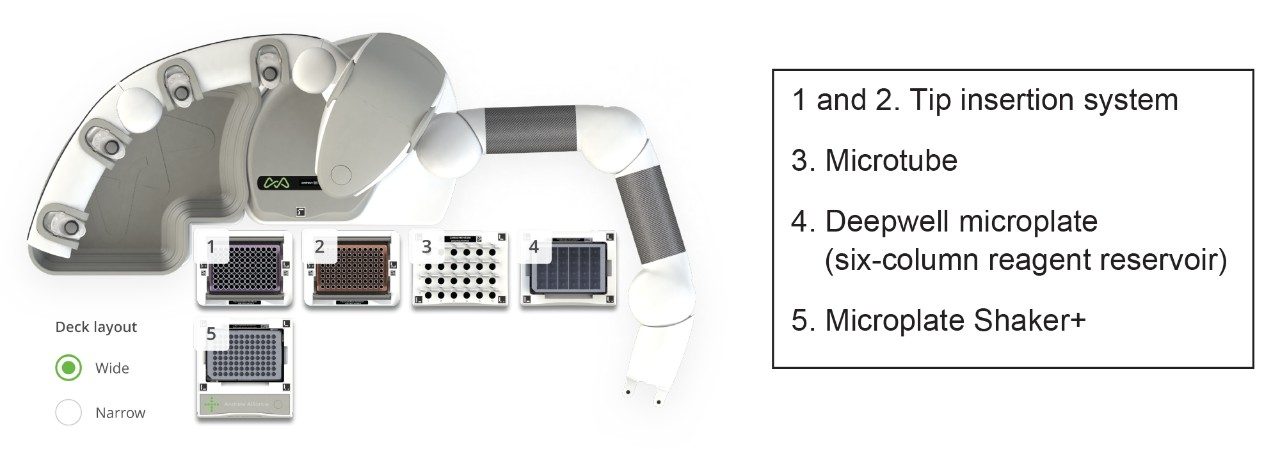
The full Andrew+ deck layout for both scripts can be seen in Figures 1 and 2.

A manual preparation was also performed to compare the recovery and the precision of the automated workflow versus manual workflow.
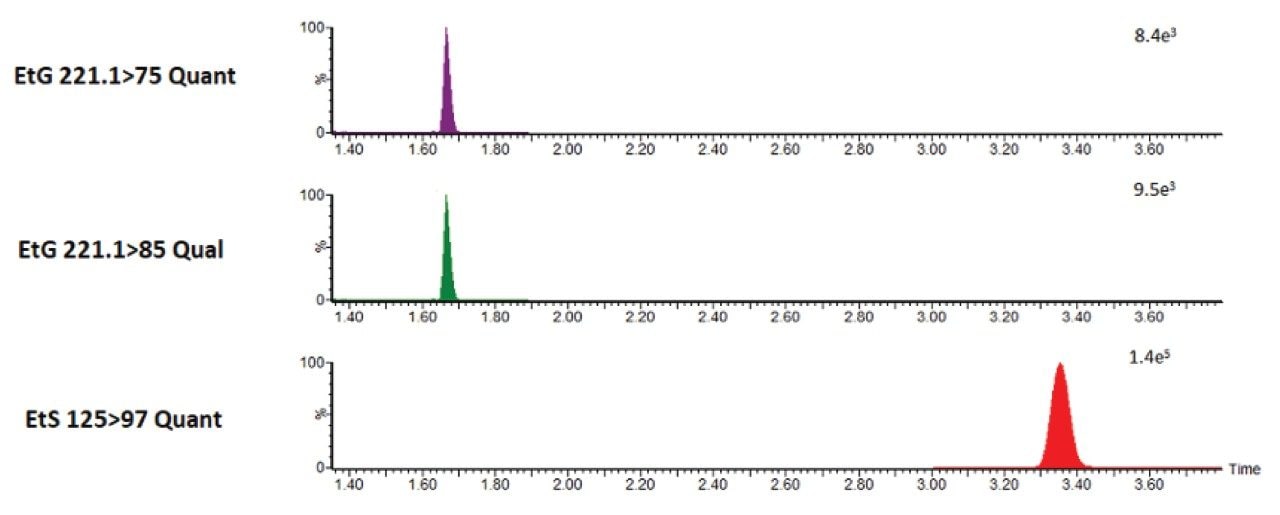
Following sample preparation, multiple reaction monitoring (MRM) was performed using the previously specified transitions documented in Waters Application Note 720006273. Two transitions were used for EtG and one transition for EtS. For EtG, a target quantifier/qualifier ratio was adopted using quality control concentration EtG/EtS:500/250 ng/mL.
Figure 3 is a representation of an automated injection. Results are equivalent to what was achieved in Waters Application Note 720006273. Acceptability criteria included +/- 20% of target ion ratio.
The precision of all methods was assessed at two concentrations for EtG (200, 500 ng/mL) and EtS (800, 2000 ng/mL). The “dilute and shoot” automated assays for precision is comparable to manual preparation workflow. For EtS, the precision slightly improved. See Table 1 for precision results.

The comparison of recoveries for the automated workflows performed by the Andrew+ versus the manual workflow can be seen in Figure 4. The overall recovery of results (n = 8) achieved is +/- 2% compared to a manual workflow for both EtG and EtS, QC low and QC high samples.
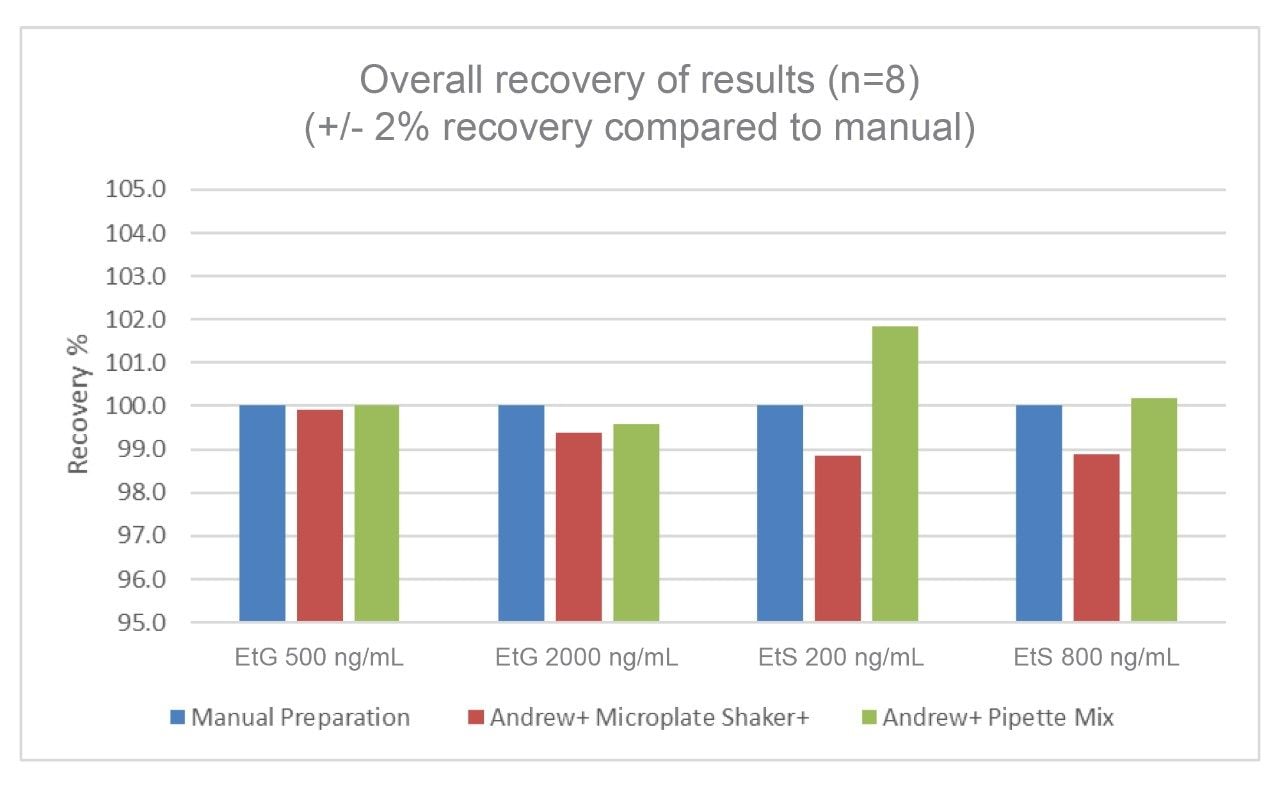
Both automated methods developed on the Andrew+ have demonstrated very comparable and equivalent performance to a manual preparation workflow. This offers customers the option to use either automated methods for the quantification of EtG and EtS in human urine. In a high throughput setting workflow, efficiencies would be gained using the Shaker+ Microplate as the option for pipette mixing.
For further information on materials and LC-MS/MS methods used, refererence protocol 720006273.
Automated methods for dilute and shoot sample preparation of EtG and EtS are demonstrated using the Andrew+ Pipetting Robot in a high throughput setting. The Andrew+ has demonstrated that mixing of samples can be performed by Pipette mixing on Andrew+ or the Microplate Shaker+.
The developed methods are accurate, precise, and comparable to manual preparation for the identification and quantification of biomarkers EtG and EtS.
720007186, March 2021