This is an Application Brief and does not contain a detailed Experimental section.
For research use only. Not for use in diagnostic procedures.
This work demonstrates the automated extraction of MMA from serum employing the Ostro Plate for protein precipitation and phospholipid removal on the Andrew+. The development of the MMA Andrew+ protocol takes advantage of the existing MMA workflow and LC-MS analytical method which allowed for the adoption of high throughput sample preparation. Utilizing the Andrew+ for MMA sample preparation enables flexible and reproducible sample preparation while sparing analyst time.
Sample quantification is performed on an ACQUITY UPLC I-Class System using an ACQUITY UPLC CSH C18, 1.7 μm Column (p/n: 186005297) with detection on a Waters Xevo TQ-S micro Tandem Quadrupole Mass Spectrometer. Using proven LC-MS/MS methods in conjunction with the Andrew+ facilitates accurate and robust analyte quantification.
The analysis of methylmalonic acid (MMA) can be used to determine the status of Vitamin B-12 serum levels. MMA is a preferable indicator to vitamin B12 due to the possibility of normal or high vitamin B-12 serum levels even in a deficient condition.1 The use of LC-MS/MS analysis for research into this biomarker is ideal for high throughput. To further facilitate the analysis of serum samples, the previously simplified MMA workflow has been automated on the Andrew+.2 Waters methylmalonic acid method utilizes the Ostro Protein Precipitation and Phospholipid Removal 96-well Plate (p/n: 186005518) which allows for cleaner, reproducible extracts in a simplified, higher throughput format.
The development of methods for automation can be seen as a daunting task even when using a simplified workflow. The Andrew+ MMA protocol has been created in the user-friendly cloud native OneLab Software which guides the user with helpful hints and drag and drop features. The MMA protocol captures the current manual preparation steps which include reagent transfer and thorough mixing within the Ostro Plate before sample cleanup on the vacuum+ device. A user prompt is present in the protocol to allow for an evaporation step to take place off deck, this allows the user to continue the protocol with the final pipetting step, once the plate is ready. The simple process of protocol assembly enables the user to focus in on parameters such as liquid handling, required for accurate pipetting and thorough mixing. The correct adjustment of these parameters can be crucial to ensuring the accuracy and precision of the sample preparation. The adjustment of the liquid handling parameters is straightforward in OneLab, allowing the user to capture the intricacies of manual pipetting. One example of an adjustment for automated preparation is the use of pipette mixing in place of a vortex. The mixing speeds were optimized to ensure a thorough mix of the sample to improve precision and recovery results.
Automation can remove the bottle neck that can result from high throughput sample preparation. When used in combination with LC-MS/MS analysis, this can enable an efficient, robust, and analyst time-saving solution. Additionally, the user-friendly software and uniform deck setup enables easier creation and performance of methods, especially when transferring from one lab to another.

When developing the protocol, improvements were made to the pipette liquid handling parameters to ensure smooth and consistent transfer of samples and thorough mixing within the Ostro Plate before extraction. A user prompt was added to the protocol for the evaporation step which was completed off deck for around 50 minutes. An additional suggestion is that the protocol could be split into two parts at this point to allow use of the Andrew+ in the meantime, if required. The deck configuration for this protocol can be seen in Figure 1.

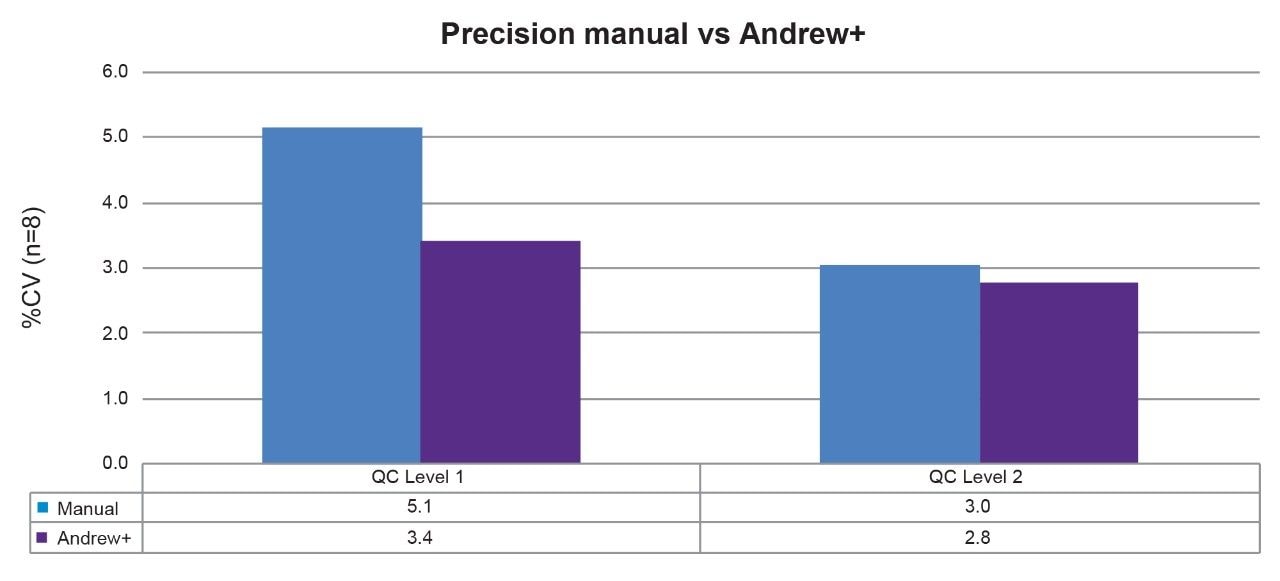
The Andrew+ transferred a set of eight aliquots of a Recipe MMA quality control Level 1 and quality control Level 2 to each of the first two rows of the 2 mL collection plate (p/n: 186002482). The same preparation was performed manually, as a comparison. The area counts of the eight sample preparations at each level for Andrew+ were compared to a manual preparation. Improved precision was demonstrated on the Andrew+ in comparison to manual with eight preparations at both QC levels ≤3.5% for automation and ≤5.2% for manual preparation (see Figure 2).
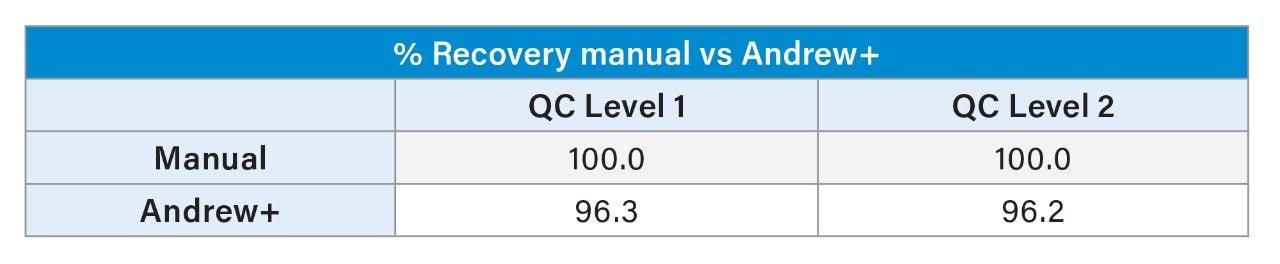
The %Recovery in area counts was also calculated for Andrew+ against manual. A difference of <4% between manual and automated preparation was observed at both QC levels, demonstrating the comparability of Andrew+ to manual.
Further information on the MMA UPLC-MS/MS method and materials used can be found in the Waters Application Note 720006806.
An automated sample preparation for MMA has been developed successfully using Andrew+. The analysis of QC samples across the concentration range exhibits excellent precision and recovery in agreement with a manual preparation.
720007187, March 2021