Batch-to-Batch Robustness of MaxPeak Premier Columns for the Analysis of Dexamethasone Phosphate and Related Compounds
Abstract
An Ultra High Performance Liquid Chromatography method that uses an Arc Premier System with MaxPeak Premier Columns was used to evaluate the batch-to-batch reproducibility of multiple columns. Different batches of both the construction material and the packing material of MaxPeak Premier XBridge BEH C18 and XSelect HSS T3 were studied. Results showed that MaxPeak Premier Columns were very reproducible when used for the analysis of a mixture of metal chelating and non-metal chelating compounds. Various chromatographic parameters including relative retention time, critical pair resolution, and peak area were all investigated for reproducibility on the different columns. The columns showed excellent reproducibility for all the studied chromatographic parameters. For example, the %RSD for the peak areas for all peaks was always in the range of 0.1%–5.6% for all analytes. These findings indicate that the batch to batch reproducibility of MaxPeak Premier Columns is very high and these columns are very robust.
Benefits
- Batch-to-batch reproducibility of the construction material of MaxPeak Premier XBridge BEH C18 and XSelect HSS T3 Columns
- Batch-to-batch reproducibility of the packing material of MaxPeak Premier XBridge BEH C18 and XSelect HSS T3 Columns
Introduction
Stainless-steel has widely been used as material to build liquid chromatography instruments and columns because of its unique properties of corrosion resistance1 manufacturability, and inertness. However, some classes of analytes such as metal chelating compounds can interact with metal oxide films because of the electron deficient nature of these metal ions. For example, phosphorylated analytes can readily adsorb to the electron deficient surfaces of stainless-steel within the flow path of the chromatographic system. Such interactions can result in poor chromatographic peak shape, severe analyte losses, and quantitative inaccuracies.2,3
To address this, Waters has recently developed a family of technologies named MaxPeak High Performance Surfaces (HPS). These surfaces are composed of a highly crosslinked layer related to that of ethylene-bridged hybrid (BEH) chromatographic particles. The MaxPeak HPS Surfaces are designed to increase analyte recovery, sensitivity, and reproducibility by mitigating undesired interactions with metal surfaces. Column reproducibility is a key parameter that has a critical impact on the long-term reliability and robustness of analytical methods. This is because column-to-column and batch-to-batch variability can result in unacceptable chromatographic performance that could require the method to be revalidated for regulatory acceptance. As such, it is essential that the selected columns are rugged and reproducible when developing analytical methods to reduce the risk of having out-of-specification and out-of-trend results throughout the method life.
The main goal of this study is to investigate the lot to lot reproducibility of three different XBridge BEH C18 Columns that were constructed using different lots of High Performance Surfaces material. Additionally, the reproducibility of three different stationary phase batches of XBridge BEH C18 Columns with the same lot of High Performance Surfaces material will also be examined here. The same study will be performed on XSelect HSS T3 Columns, too. To do this a UHPLC method that has previously been developed for the analysis of metal-sensitive pharmaceuticals/related compounds (See Figure 1 for structures) will be used to evaluate the different XBridge BEH C18 and XSelect HSS T3 Columns.4
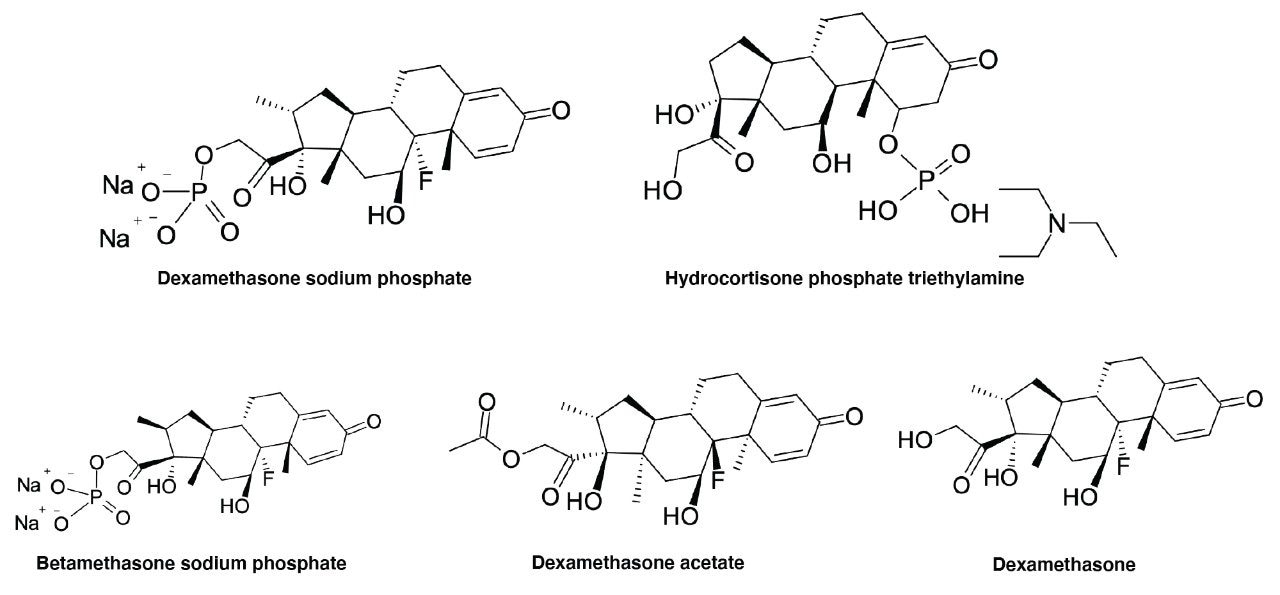
When looking at the repeatability of chromatographic parameters using different batches of columns, variances may occur in retention times, peak areas, peak symmetries, efficiencies, and various other parameters. Reproducibilities of the retention and several other profile characteristics of the peaks obtained with these different columns will be evaluated.
Experimental
Hydrocortisone phosphate triethylamine, dexamethasone sodium phosphate, betamethasone sodium phosphate, dexamethasone, and dexamethasone acetate were all purchased from the United States Pharmacopeia (USP) (Rockville, MD, USA). Stock solutions of these compounds were prepared by accurately weighing the desired amounts of each standard and dissolving them in 50/50 (v/v) water/acetonitrile solvent. The stock solutions were then used to make a test mixture that contains the two APIs and three dexamethasone phosphate related compounds. This mixture was prepared by diluting the stock solutions of each one of the standards in 90/10 (v/v) water/acetonitrile as sample solvent. The final concentration of each analyte in the test mixture were approximately: 0.1 mg/mL Hydrocortisone phosphate triethylamine, dexamethasone sodium phosphate, and 0.07 mg/mL for each related compound.
LC Conditions
|
LC system: |
Arc Premier with Quaternary Solvent Manager (rQSM), Sample Manager (rFTN), Column Manager, and a CM Aux, PDA Detector, ACQUITY QDa Mass Detector |
|
Detection: |
PDA |
|
Column(s): |
MaxPeak HPS XSelect HSS T3, 4.6 × 100 mm, 2.5 µm pH range: 1–10 MaxPeak HPS XBridge BEH C18, 4.6 × 100 mm, 2.5 µm pH range: 1–10 |
|
Column temp.: |
35 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
3 µL |
|
Flow rate: |
0.5 |
|
Mobile phase A: |
10 mM Ammonium formate in water |
|
Mobile phase B: |
Acetonitrile (0.1% Formic acid) |
|
Gradient: |
10 to 90% B/5 or 15 min* Gradient starts at t=0 and a final hold of 2 minutes was applied before returning to initial conditions. |
|
UV detection: |
254 nm |
MS Conditions
|
MS system: |
ACQUITY QDa Mass Detector |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
100–500 Da |
|
Capillary voltage: |
0.8 kV |
|
Source temperature: |
600 °C |
|
Cone voltage: |
15 V |
Data Management
|
Chromatography software: |
Empower 3 Chromatography Data System |
Results and Discussion
Relative Retention Time
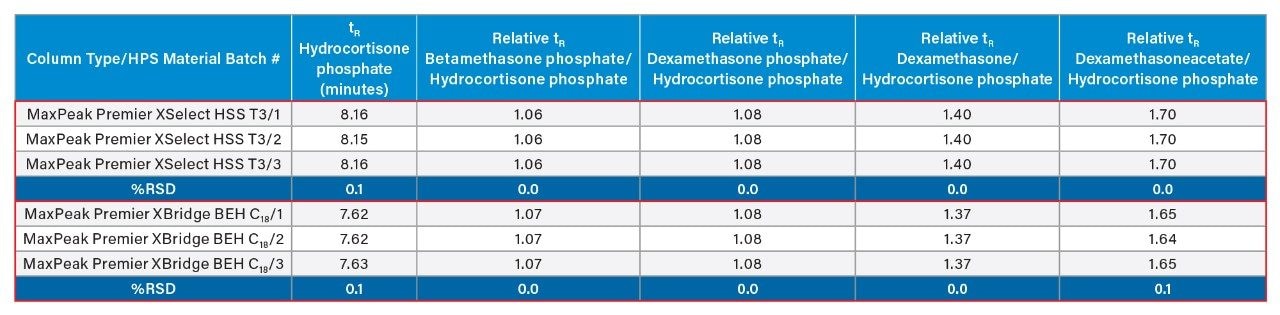
A key parameter to consider when evaluating batch-to-batch reproducibility of columns is the relative retention time of compounds. Table 1 summarizes the relative retention times of dexamethasone phosphate and its related compounds (referenced to hydrocortisone phosphate) when analyzed on different batches of HPS material for the MaxPeak XSelect HSS T3 and the MaxPeak XBridge BEH C18 Columns. Results showed that the relative retention time is very reproducible on the different batches for all the analytes on both column chemistries. For example, the %RSD for the relative retention times of dexamethasone phosphate and its related compounds were always ≤0.1% on the different batches of HPS material for both column chemistries. These results indicate that the in-house specialized manufacturing process for the HPS material is highly precise.
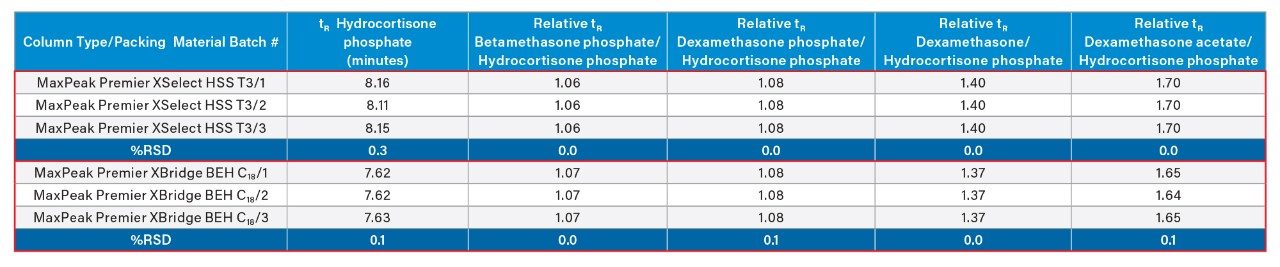
It should be mentioned here that reproducibility of different batches of packing material (with the same lot of the construction material) was also studied here. Results showed, as can be seen in Table 2, that the relative retention times for dexamethasone phosphate and its related compounds were also remarkably reproducible with %RSD values of ≤0.1% for all analytes when injected on three different batches of packing material for both column chemistries.
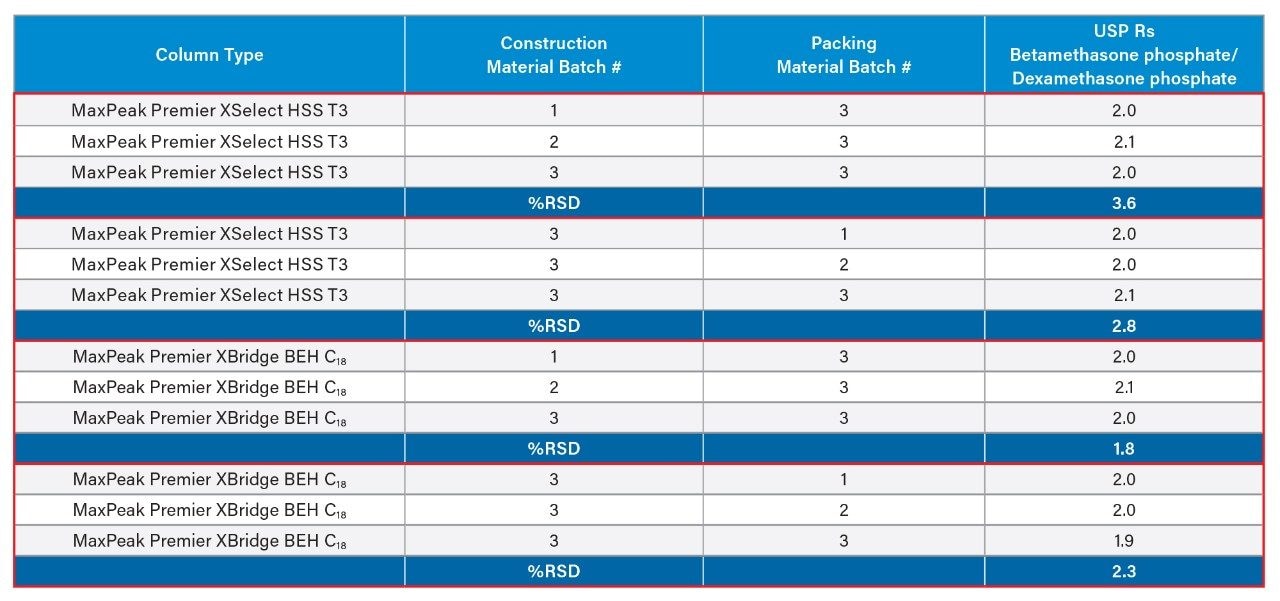
Critical Pair Resolution
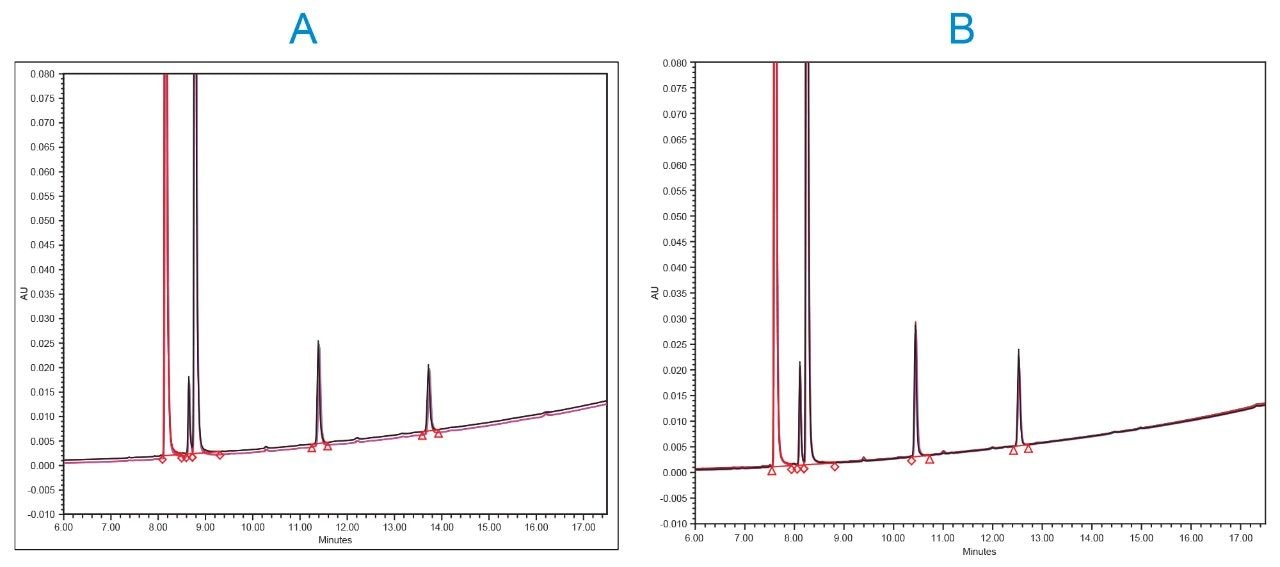
Another chromatographic parameter that is important to consider when evaluating column-to-column reproducibility is the resolution of the “critical pair”. A critical pair represents the two components of the chromatogram with the lowest calculated resolution between them. In this case, our critical pair was betamethasone phosphate/dexamethasone phosphate. The ability of the different columns to reproducibly resolve the critical pair was also evaluated here for both the different batches of HPS material and the different packing material batches. Results revealed that all columns for both chemistries were able to resolve these two components very reproducibly as can be seen in Table 3. Representative chromatograms of dexamethasone phosphate and related compounds on the different batches of the construction material for the two column chemistries used in this study are depicted in Figure 2.
Peak Area
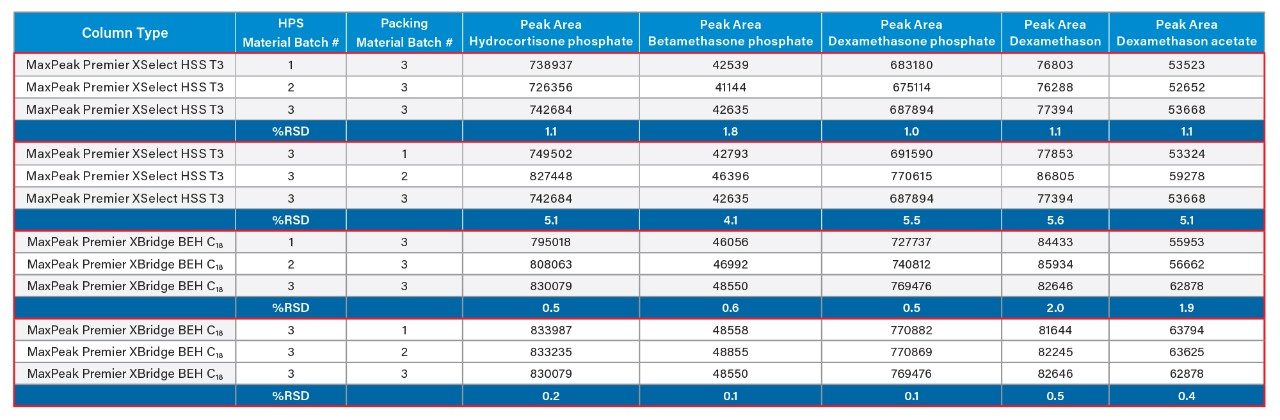
Consistency in peak area when the same amount of sample is injected onto different columns is also another key parameter to consider when studying batch-to-batch reproducibility. This is particularly important for quantification purposes when consistency in area is key for obtaining correct results. Table 4 shows the peak areas of Dexamethasone phosphate, related compounds, and hydrocortisone phosphate when analyzed on the different columns tested in this study. The peak area of all analytes showed excellent reproducibility across the different batches of the construction material as well as the different batches of packing material.
Conclusion
- This application note clearly demonstrates the robustness of MaxPeak Premier XSelect HSS T3 and XBridge BEH C18 Columns when used for the analysis of dexamethasone phosphate and related compounds.
- Using high batch-to-batch reproducibility MaxPeak Premier Columns for analytical methods is very advantageous especially for methods that are used for a long time.
- This application note also demonstrates the excellent control of the column temperature, the mobile phase flow-rate, and the mobile phase composition obtained with the Waters equipment.
References
- S.F. Cogan, G.S. Jones, D.V. Hills, J.S. Walter, L.W. Riedy. Comparison of 316LVM and MP35N Alloys as Charge Injection Electrodes, J. Biomed. Mater. Res. 28(2) (1994) 233–40.
- T.H.W. M. Lauber, M. DeLano, C. Boissel, M. Gilar, K. Smith, R. Birdsall, P. Rainville, J. Belanger, and K. Wyndham. Low Adsorption UPLC Columns Based on MaxPeak High Performance Surfaces, Waters White Paper, 720006930EN, 2020.
- K.E. Collins, C.H. Collins, C.A. Bertran. Stainless Steel Surfaces in LC systems, Part II: Passivation and Practical Recommendations, LC GC North America 18(7) (2000) 688–692.
- F.L. Alkhateeb, P.D. Rainville. Analytical Quality by Design Based Method Development for the Analysis of Dexamethasone Phosphate and Related Compounds Using Arc Premier MaxPeak High Performance Surfaces (HPS) Technology, Waters Application Note, 720007272EN, 2021.
720007350, August 2021