This is an Application Brief and does not contain a detailed Experimental section.
For research use only. Not for use in diagnostic procedures.
In this application brief we demonstrate the ability of Cyclic IMS to separate small molecule isomers by utilizing multi-pass experiments, providing improved metabolite detection and identification.
Multi-pass experiments using the Cyclic IMS (cIMS) Mass Spectrometer highlight enhanced mobility separation of structural isomers.
Over the years, advances in separation technology have enabled the detection and quantification of compounds that have previously proved difficult to analyze. In metabolomics, many small compounds – which form the core of the analytes of interest fall into this group. The variation in compound modifications during metabolism can generate subtly different isomeric metabolites. These isomers can each tell a very different story when it comes to furthering the understanding of diseases and ultimately biochemical mechanisms. Chiral separation of isomers by supercritical fluid chromatography (SFC) has to some degree allowed these separations to be performed in short analytical acquisitions.1,2 Nevertheless, SFC requires a separate chromatography system to traditional liquid chromatography (LC) and therefore lacks the method flexibility associated with the latter configuration.
Ion mobility enabled mass spectrometers provide an additional orthogonal separation of ions prior to detection. This allows molecules of similar mass/charge to be separated by their collisional cross section (CCS) improving specificity, enabling improved identification, and cleaner MS/MS spectra. Although, some very closely related structural isomers exhibit the same or similar CCS value and drift time when measured on conventional ion mobility platforms, accurate determination is difficult by this method alone.
The SELECT SERIES Cyclic IMS instrument has previously been used to assist in the resolution of larger molecules such as peptides, polysaccharides, and lipids3 by performing multi-pass IMSn acquisitions (IMSn) where packets of ions can be cycled multiple times around the IMS region before being ejected for detection. In this application brief we demonstrate the Cyclic IMS instrument’s capability of enhancing the separation of small polar isomeric metabolites using the multi-pass feature.
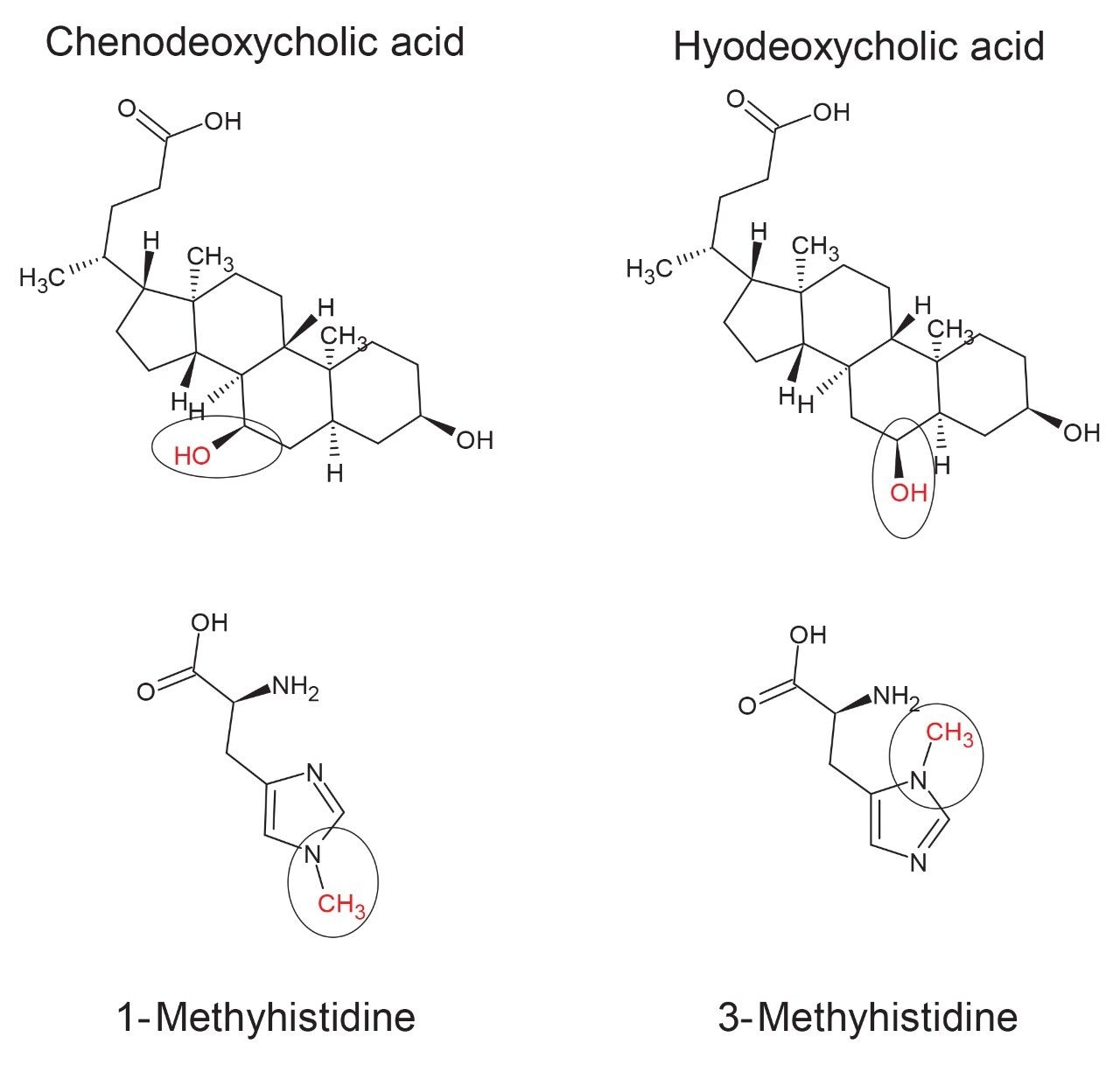
Isomeric forms of amino acids and bile acids exist among others – which ultimately play significantly different biological roles – highlighting the importance of a metabolomic analytical study in differentiating between different isomers. Methylhistidines have previously been identified as markers associated with a variety of diseases and deficiencies, including vitamin E deficiency, which has been shown to lead to an increase in the presence of 1-methylhistidine in urine.4 Conversely, an increase in plasma concentrations has been correlated to a reduction in carnosinase activity. Furthermore, 3-methylhistidine has been identified as a potential marker for the breakdown of skeletal muscle following injury.5 Utilization of an in-house CCS prediction tool provided predicted CCS values for 1-methylhistidine and 3-methylhistidine as 133 Å2 and 134 Å2 respectively ([M-H]- ion), indicating potential difficulties in accurately confirming these compounds.
Bile acids play import roles in lipid and fat-soluble vitamin adsorption, acting as surfactants, and are intrinsic in cholesterol metabolism.6,7 Chenodeoxycholic acid is a primary bile acid in humans which undergoes a biotransformation upon interaction with gut bacteria to produce secondary bile acids (e.g. hyodeoxycholic acid).7 High levels of chenodeoxycholic acid can lead to a toxic effect from cholestasis. Excretion of bile acids in mammals is performed following glucuronidation of these secondary bile acids. Due to the positioning of the hyodeoxycholic acid hydroxyl groups, this secondary bile acid has an increased rate of glucuronidation and ultimately an increased rate of urinary excretion.8 Variations in levels of these bile acids can be indicative of microbiome function. The predicted CCS values for both bile acids is 207 Å2 ([M-H]- ion).
A standard mixture containing two sets of isomeric endogenous metabolites from two different compound classes was prepared for direct infusion using acetonitrile:water (1:1) as diluent. Additionally, each individual authentic standard was prepared into separate infusion solutions for further confirmation. The standard infusion mixture contained two bile acids (chenodeoxycholic acid and hyodeoxycholic acid) and two modified histidine metabolites (1-methylhistidine and 3-methylhistidine) (Figure 1).
All mass spectrometry data was acquired using negative electrospray ionization (ESI) and the Tof operated with V optics mode. Source conditions (i.e. capillary voltage, source temperatures, and gas flows) were optimized for each compound class to provide the best response for assessing the IMS separation. In order to perform the multi-pass experiment, the Cyclic IMS instrument was operated in MS/MS mode without any transfer collision energy but with the quadrupole set to the corresponding negative molecular ion mass for the bile acids (m/z = 391.28 [M-H]-) and methylhistidines (m/z = 168.07 [M-H]-). All infusion solutions were infused directly into the source using a syringe pump set to a flow rate of 10 µL/min.
Whilst infusing the standard solutions, ions corresponding to the bile acids and methylated histidines were individually sent around the cyclic ion mobility (IM) cell multiple times until the individual isomers were separated by their arrival time (Figure 2). Baseline resolution was achieved for the methylhisitidine after 5 passes of the cyclic IM cell (Figure 2b) with the bile acids distinctly separating after 20 passes (Figure 2a). The 20 passes required to separate the bile acids was achieved in less than 200 ms at an IMS resolution of 290, whereas the 5 passes required for the methylhisitidine isomers was less than 50 ms and an IMS resolution of 145. To identify which peak corresponded to the correct isomer, the individual standards were infused to determine their specific arrival times (Figure 3).
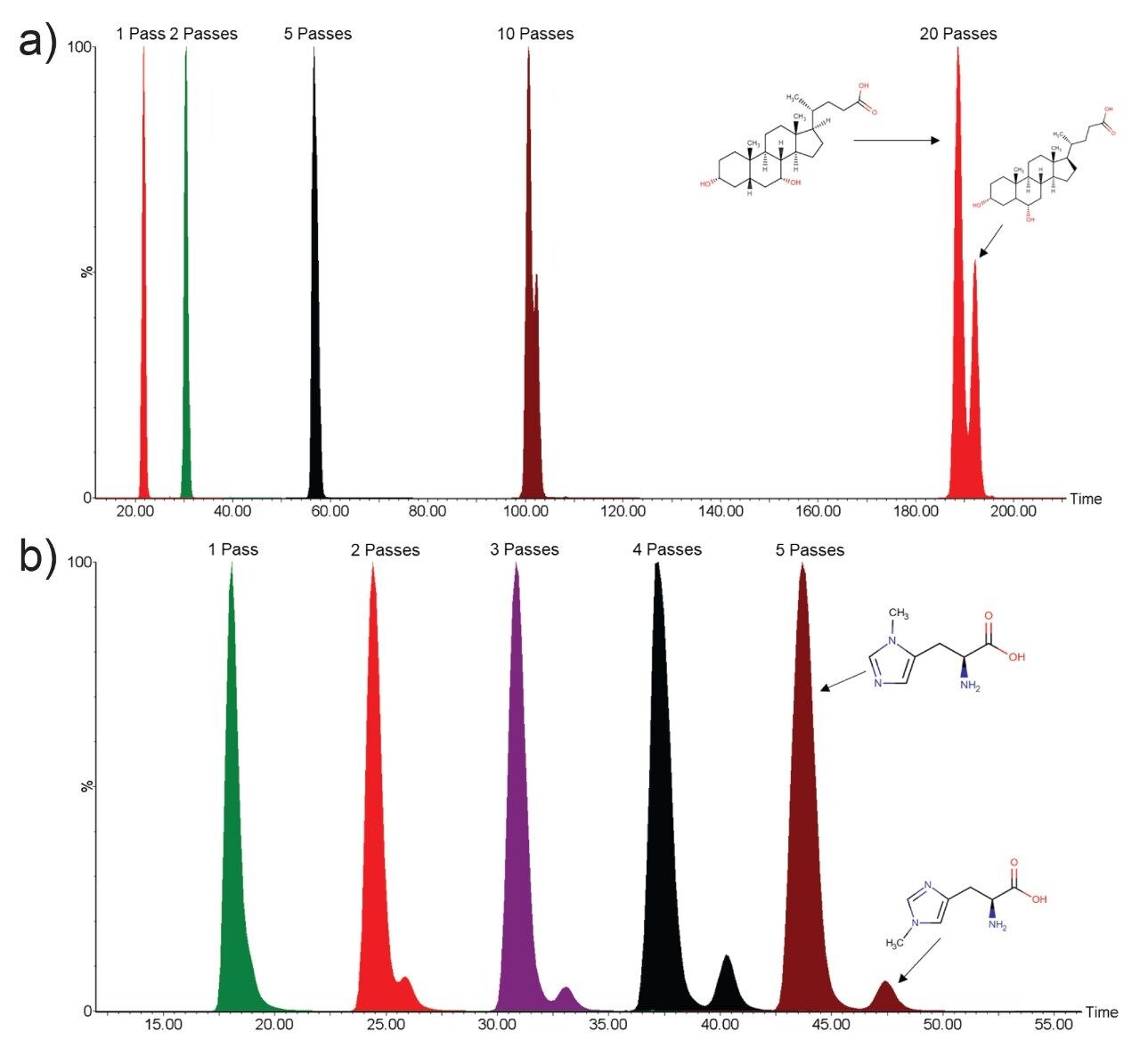
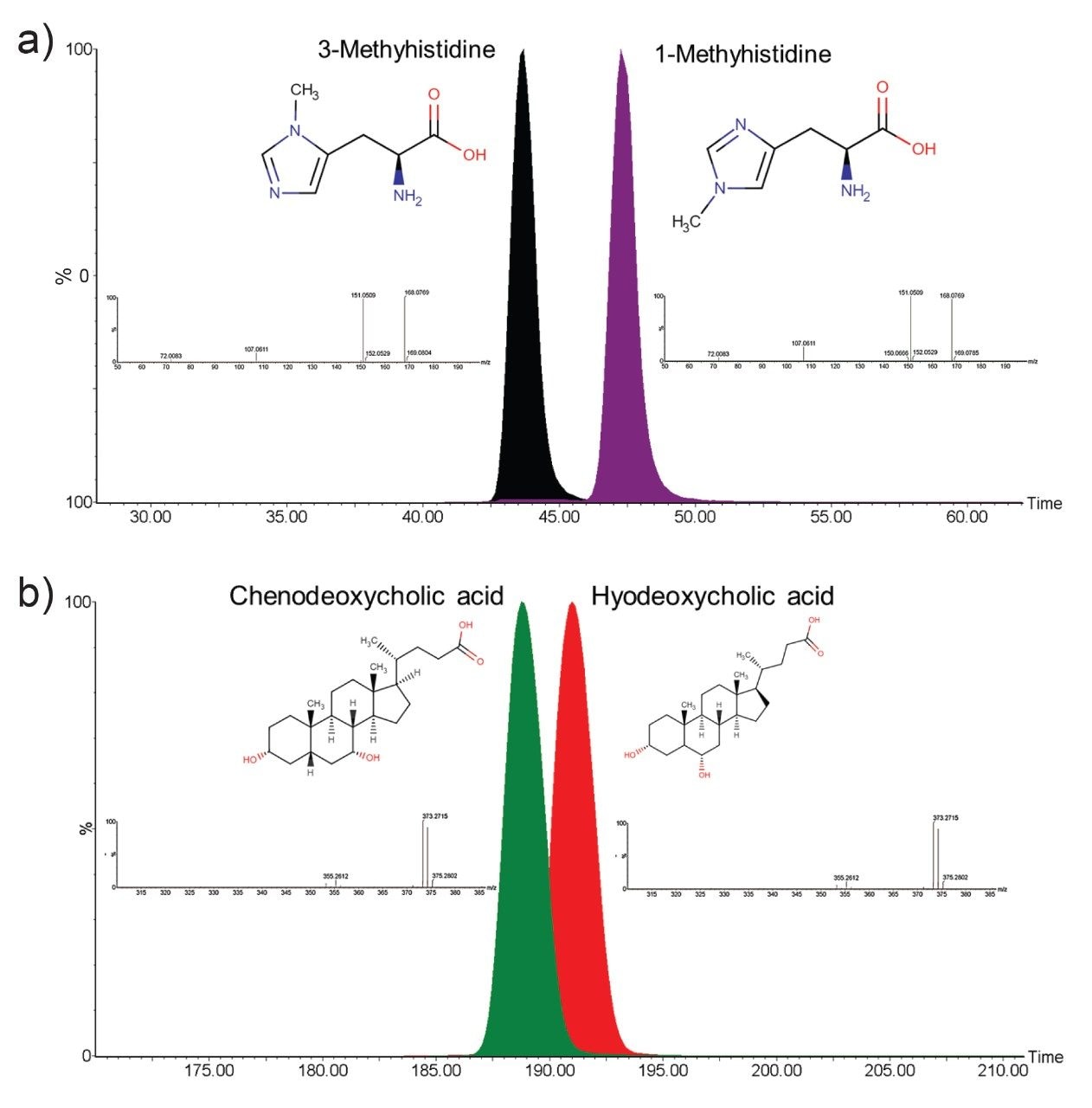
Small molecule metabolites are intrinsic in all biochemical processes as either the target of a metabolic pathway or the resulting product of metabolism. These molecules can therefore provide insights into the disfunction of pathways caused by genetic abnormalities, diseases, and the impact of lifestyle. Many compounds are present naturally in different isomeric forms which in turn can be related to different metabolic pathways and have varying impact on the underlying biology and associated health conditions. The SELECT SERIES Cyclic IMS instrument has been shown to accurately resolve two sets of biologically relevant isomers within 20 passes (<200 ms) of the cyclic IMS cell. This has enabled the isomeric forms to be resolved by different arrival times for accurate identification without the need to develop a new LC method.
720007206, April 2021