Screening and Confirmation Testing of a Counterfeit M30 Pill Extract Adulterated With Xylazine
This is an Application Brief and does not contain a detailed Experimental section.
For forensic toxicology use only.
Abstract
The presence of xylazine in counterfeit M30 pills has been a disturbing trend in drug seizures for the past two years across the United States. Here we describe a workflow to screen and confirm the substances found in a counterfeit M30 pill extract.
Benefits
- The ACQUITY™ RDa™ Detector provides a quick and efficient method to perform a comprehensive screen on seized drug samples
- The combination of accurate mass, short analysis time, a customizable and expandable library, and full scan acquisition with fragmentation provides a high confidence result for drug screening
- The Xevo™ TQ-Absolute and TQ-S micro Tandem Mass Spectrometers provide excellent sensitivity for drug confirmation analysis
Introduction
Criminal drug networks are mass-producing fake pills and falsely marketing them as legitimate prescription pills to deceive unsuspecting customers. Many fake pills are made to look like prescription drugs such as oxycodone (Oxycontin®, Percocet®), hydrocodone (Vicodin®), and alprazolam (Xanax®); or stimulants like amphetamines (Adderall®) as shown in Figure 1. These fake prescription pills are easily accessible and often sold on social media and e-commerce platforms, making them easily available, including to minors. Over the past two years across the United States there has been a disturbing trend in drug seizures where xylazine, a veterinary tranquilizer, is being used as an adulterant in an increasing number of illicit drug mixtures, and has been linked to a growing number of overdose deaths.1

Previously we have described an LC-Tof method for the screening of seized drugs using the ACQUITY RDa Detector.2 Seized drug screening utilizing the ACQUITY RDa Detector provides a quick and efficient method to screen a customizable target list of drugs and in addition facilitates the identification of unidentified compounds. Here we illustrate the use of the ACQUITY RDa Detector for seized drug screening utilizing a dissolve, filter, dilute and shoot sample preparation of a counterfeit M30 pill. An LC-MS/MS system operating in MRM detection mode will be used for confirmation of the identified components using the Xevo TQ Absolute (or Xevo TQ-S micro) Mass Spectrometer coupled to an ACQUITY UPLC™ I-Class PLUS (FTN) System. Both instruments have excellent sensitivity for this type of semi-quantitative test and provide options for different budgets.
Experimental
Sample Preparation
A counterfeit M30 pill sample extract was provided by the San Diego County Sheriff’s Department-Controlled Substance Unit. The M30 pill was prepared by shaving to produce a powder and approximately 1 mg of powder was dissolved in 1 mL of ethanol prior to filtering with a 0.2 µm PTFE Acrodisc Syringe Filter (Waters p/n: 186009327).
Three drops of the filtered solution were diluted in 20% aqueous methanol (Screening Solution) prior to injecting 10 µL onto the UPLC system for screening analysis.
The screening solution was further diluted 1 in 100 using 80/20 (v/v) water/methanol prior to injecting 1 µL onto the UPLC system for confirmation analysis (Confirmation Solution).
Seized Drug Screening Using the ACQUITY RDa Detector
A 10 µL injection of the Screening Solution was performed using an ACQUITY UPLC I-Class PLUS FTN System. Compounds were separated using ACQUITY HSS T3 1.8 µm, 2.1 x 100 mm (p/n: 186003539), with mobile phases of 5 mM ammonium formate pH 3.0 (aq) and acetonitrile with 0.1% formic acid, over a run time of 9.5 minutes. The ACQUITY RDa Detector was used to detect the compound in positive ion, full scan data (50–2000 m/z) with fragmentation. The seized drug screening experiment utilizes the full scan data with fragmentation to facilitate compound identification. A custom target list (library) of 400 compounds was used for the seized drug screening experiment. The waters_connect™ Software was used to quickly search the customizable library to identify case work samples by utilizing accurate mass, retention time and compound fragmentation data, as described previously.2
Seized Drug Confirmation and Semi-Quantitation Using Tandem Mass Spectrometry
A semi-quantitative experiment was performed on the M30 pill for eleven of the fourteen identified compounds based on standard availability. A 1 µL injection of the Confirmation Solution was performed using an ACQUITY UPLC I-Class PLUS FTN System. Compounds were separated using ACQUITY HSS T3 1.8 µm, 2.1 x 100 mm (p/n: 186003539), with mobile phases of 5 mM ammonium formate pH 3.0 (aq) and acetonitrile with 0.1% formic acid, over a run time of 4.5 minutes. Detection and semi-quantitation of the compounds was performed using a Xevo TQ Absolute and a Xevo TQ-S micro Tandem Mass Spectrometer operating in positive ionization and MRM detection mode using quantitative and qualitative transitions, as shown in Table 1.
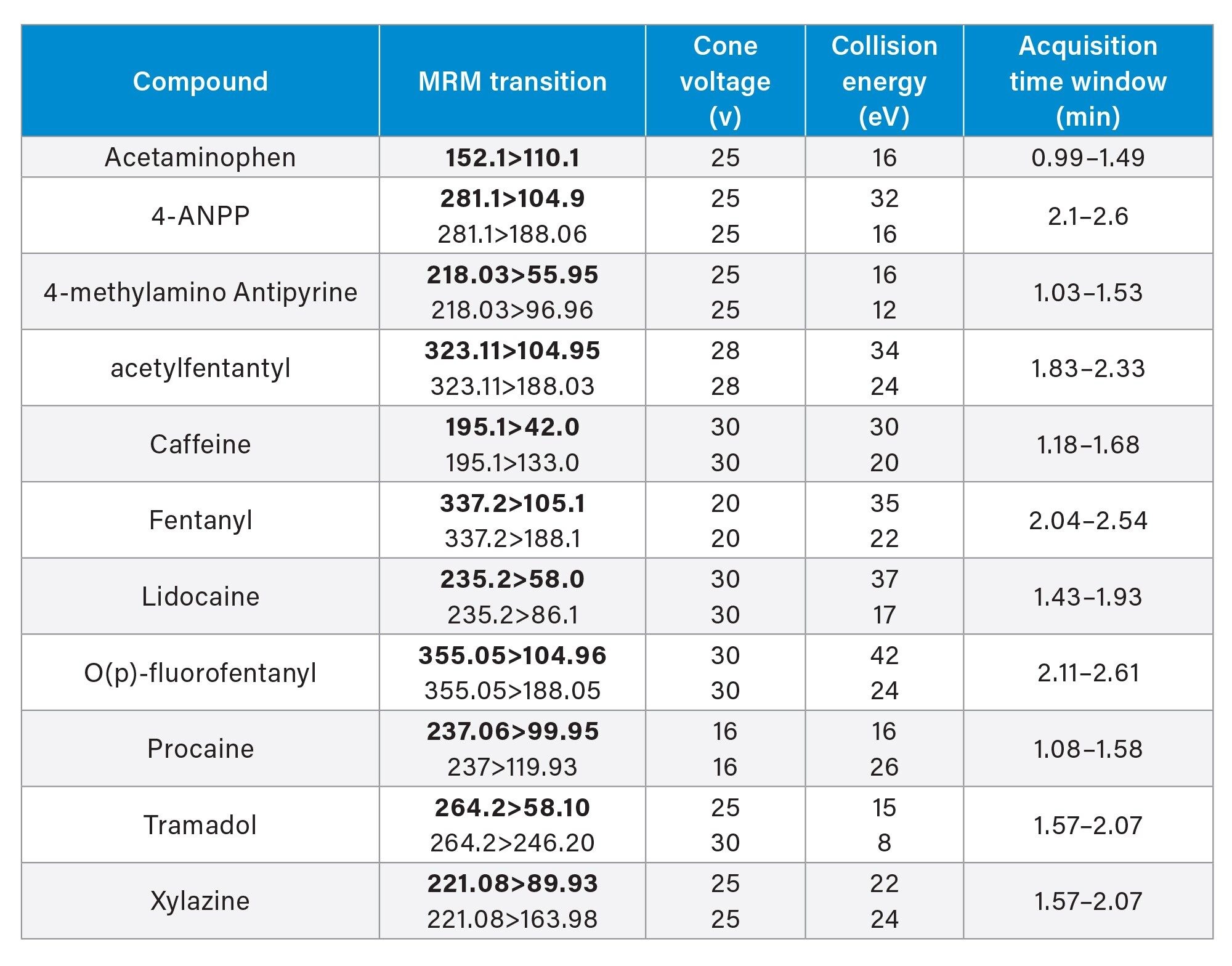
A five-point calibration curve was generated for each compound (except for acetaminophen and caffeine) ranging from 0.5 to 100 ng/mL in 20% aqueous methanol, and a quality control was prepared at a concentration of 35 ng/mL. Acetaminophen and caffeine utilized calibration curves ranging from 1.0 to 250 ng/ml with a quality control standard at 75 ng/ml in 20% aqueous methanol.
External standard quantification was employed for this analysis since the deuterated internal standards for the compounds analyzed were not available at the time of analysis.
Results and Discussion
Seized Drug Screening Using the ACQUITY RDa
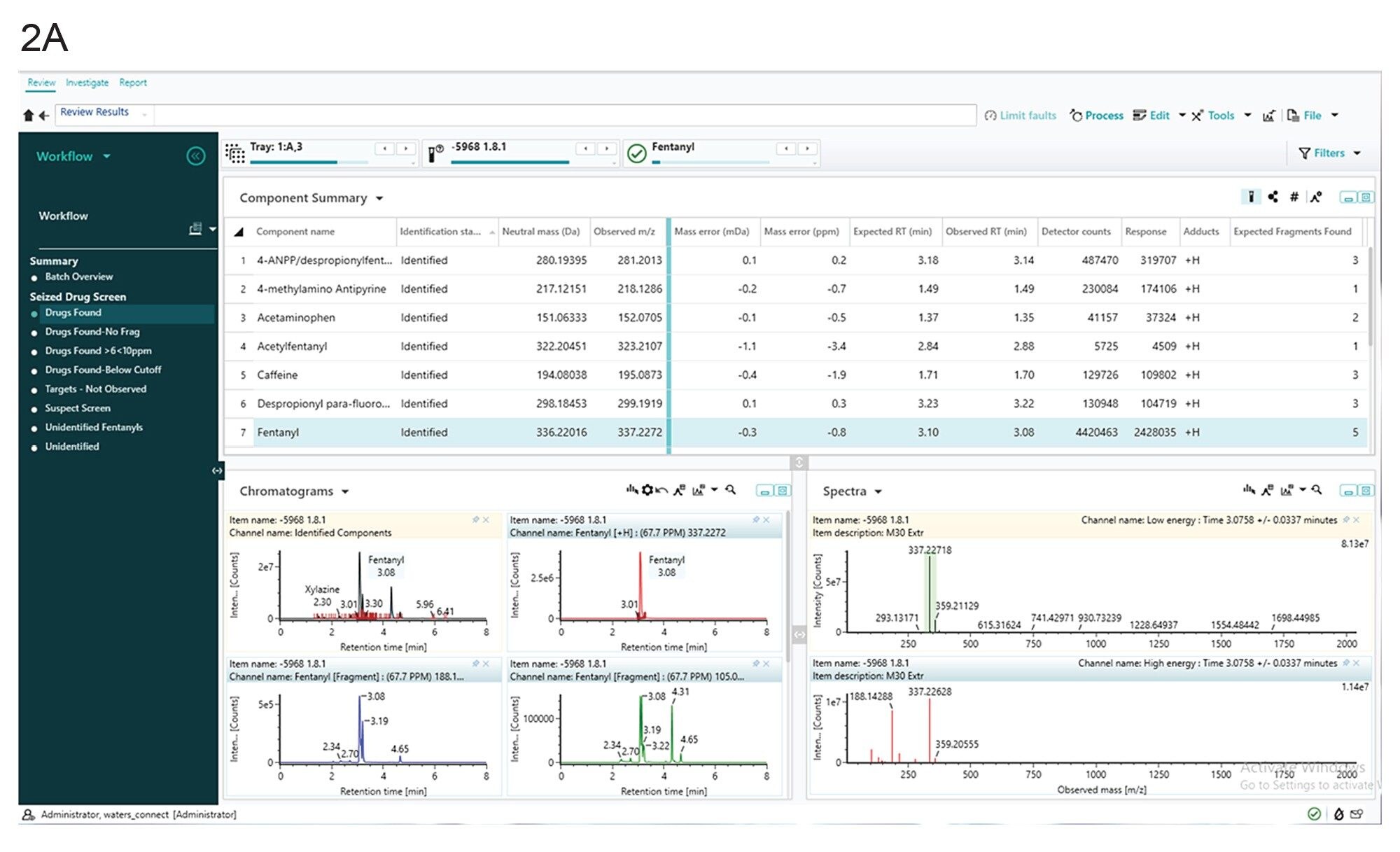
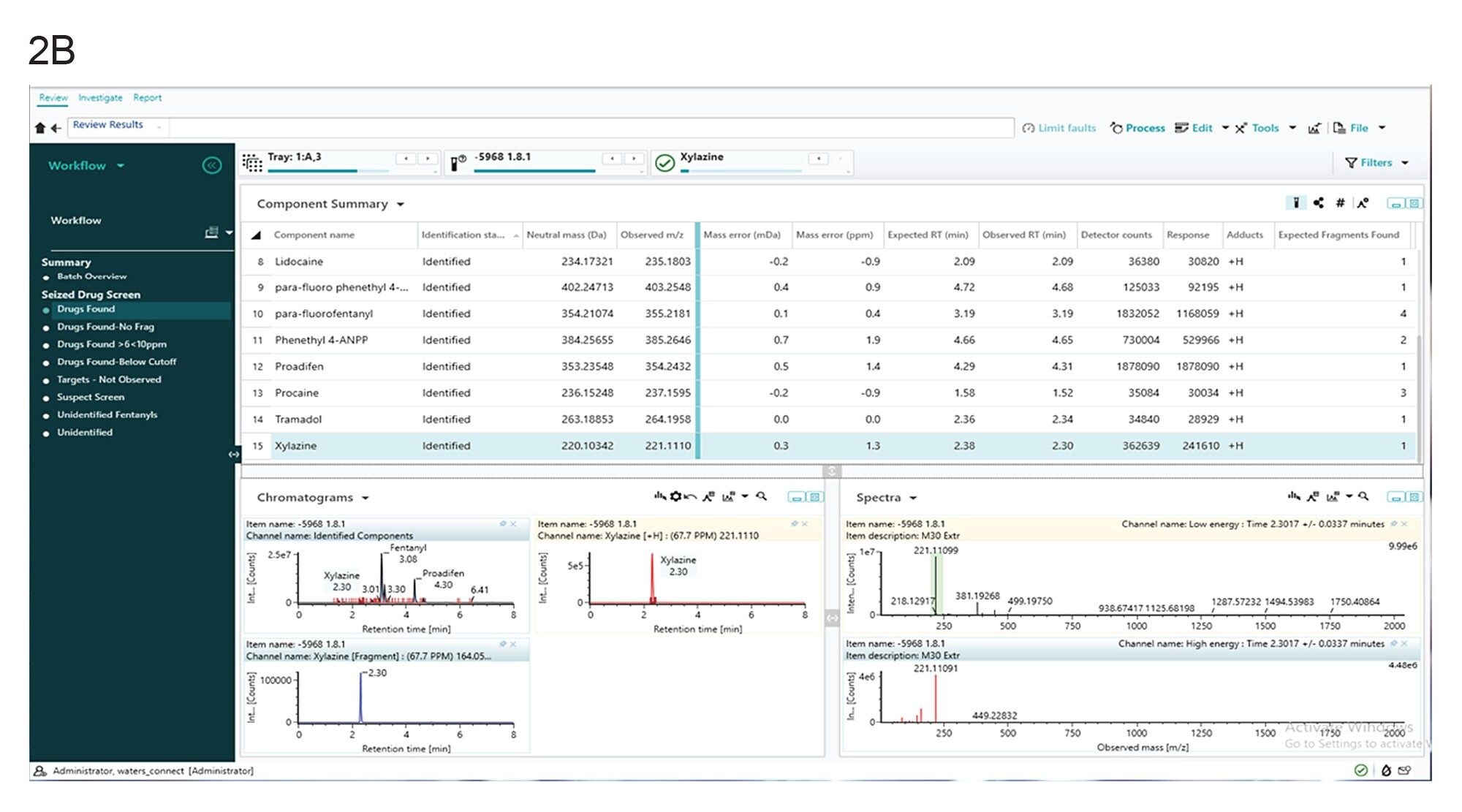
The screening method identified fourteen presumptive positive compounds (acetaminophen, 4-ANPP, 4-methylamino antipyrine, acetylfentanyl, caffeine, despropionyl para fluorofentanyl, fentanyl, lidocaine, O(p)-fluorofentanyl, para-fluoro phenethyl 4-ANPP, pheneythyl 4-ANPP, proadifen, procaine, tramadol, xylazine) in the counterfeit M30 pill extract based on a comparison of expected and observed retention time, accurate mass and instrument intensity response. Figures 2a and 2b show the waters_connect software results obtained from the seized drug screening experiment for fentanyl and xylazine respectively. The observed mass for xylazine was m/z 221.1110 with a mass error of 1.3 ppm (0.3 mDa), and the observed retention time was 2.30 min compared to the expected 2.38 min. In addition, the observed mass for fentanyl was m/z 337.2272 with a mass error of -0.8 ppm (-0.3 mDa), and the observed retention time was 3.08 min compared to the expected 3.10 min.
Seized Drug Confirmation and Semi-Quantitation Using Tandem Mass Spectrometry
Both tandem quadrupole instruments provided comparable results for each of the compounds analyzed. Each compound provided a linear response with R2 values greater than 0.995 over the calibration ranges tested.
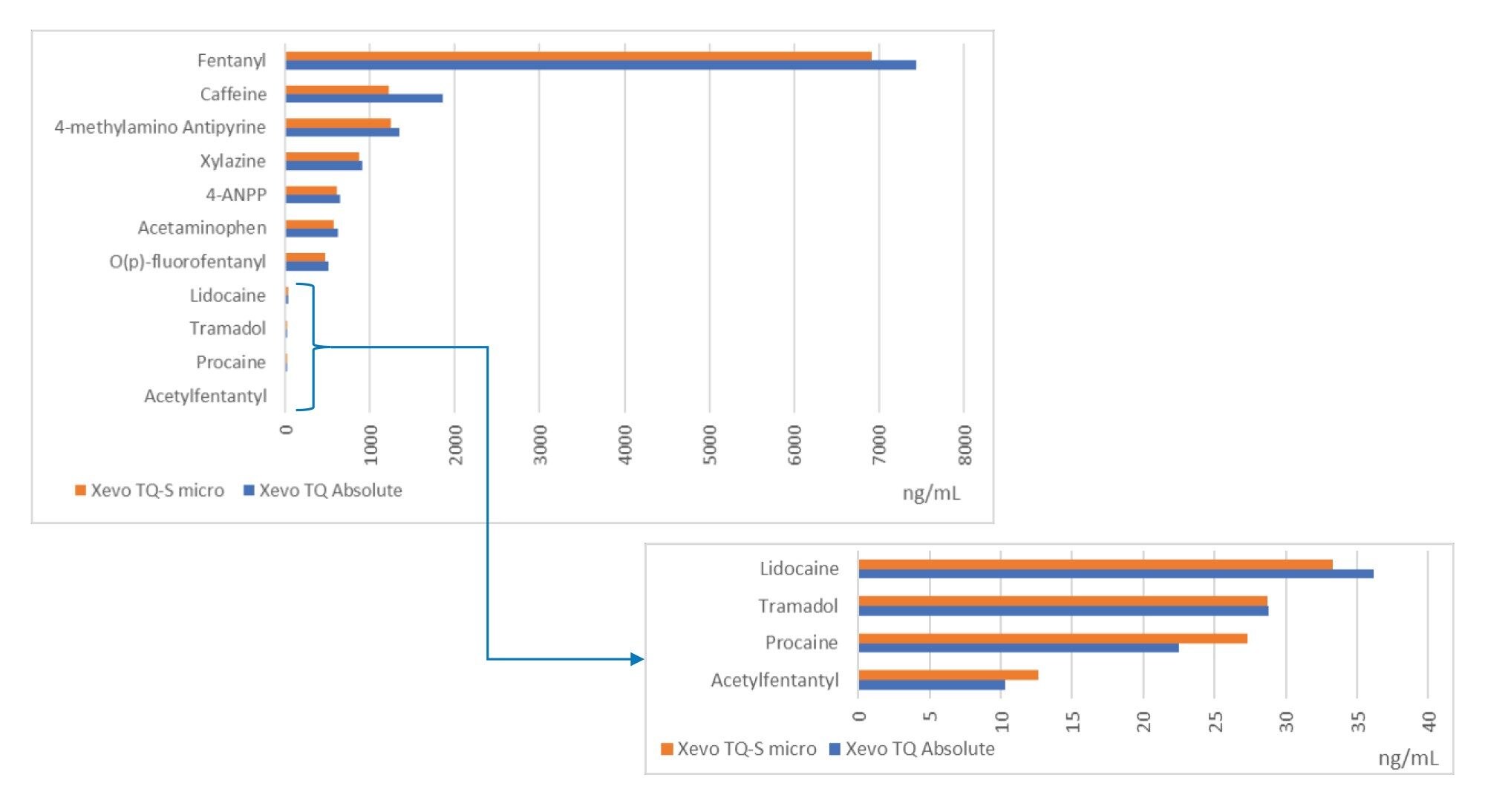
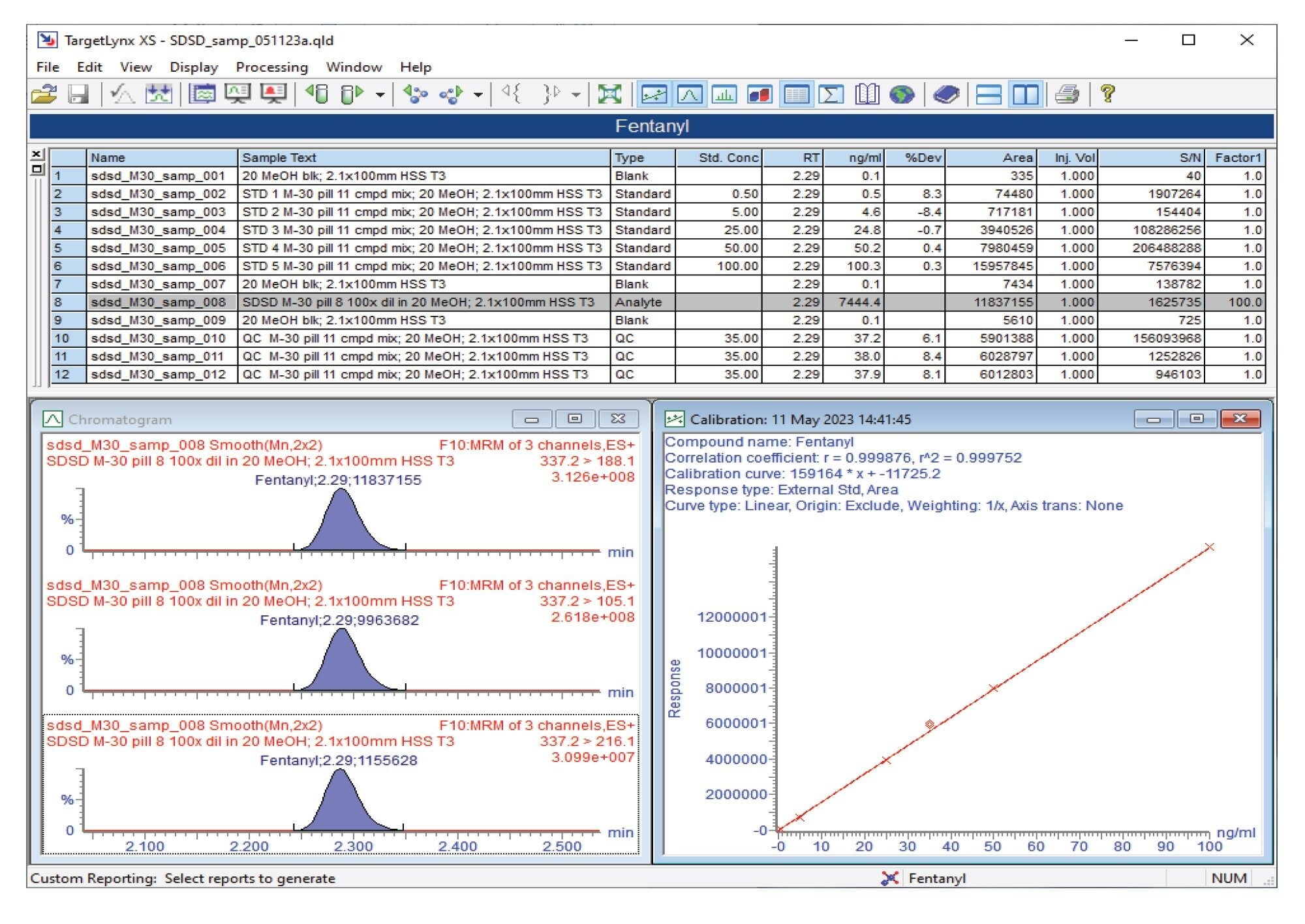
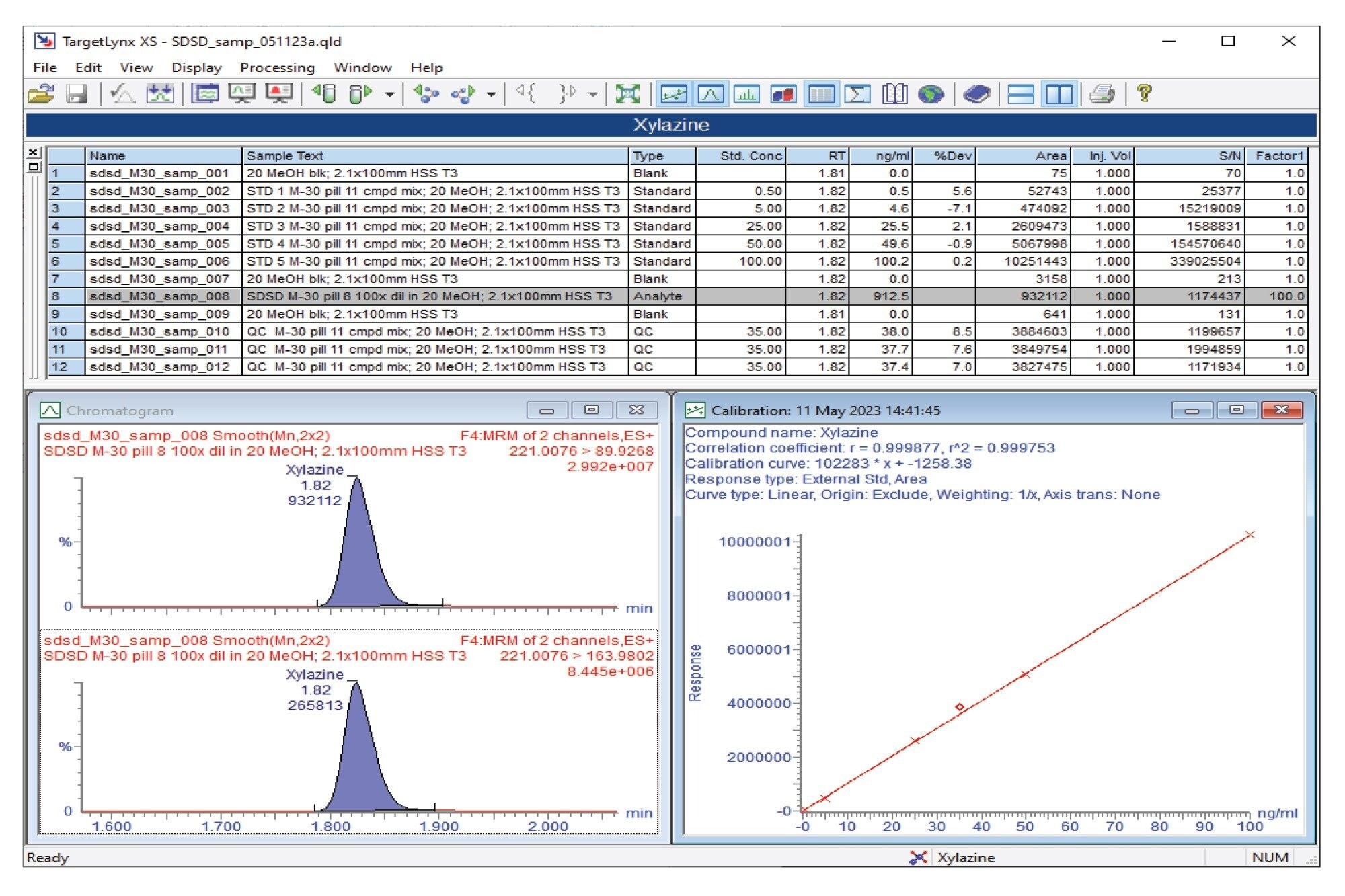
Semi-quantitative results for the M30 pill analysis obtained from the Xevo TQ Absolute and Xevo TQ-S micro are also shown in the summary table for all compounds analyzed (Figure 3). Using the Xevo TQ Absolute, fentanyl was detected at a concentration of 7444 ng/mL and xylazine at 913 ng/mL. In addition, 4-methylamino antipyrine (a metabolite of the European analgesic metamizole), 4-ANPP, O(p)-fluorofentanyl, acetaminophen, and caffeine were detected at levels above 500 ng/mL.
Figures 4 and 5 show the semi-quantitative TargetLynx™ XS results for fentanyl and xylazine, respectively, for the M30 pill using the Xevo TQ Absolute.
Conclusion
An example of a screening and confirmation workflow has been demonstrated for the analysis of a counterfeit M30 pill that was found to be adulterated with xylazine. The workflow follows identification recommendations from the Organization of Scientific Area Committees (OSAC) on seized drug analysis utilizing Categories A and B techniques to provide the highest level of selectivity through structural information and chemical and physical characteristics.
LC-Tof analysis using the ACQUITY RDa Detector provides a quick and efficient method to perform a comprehensive screen on seized drug samples. In this example, a complex mixture of fourteen compounds were presumptively identified, in a pill that should contain one active ingredient. The combination of accurate mass, short analysis time, customizable and expandable library, and full scan data acquisition with fragmentation, provides a high confidence result for the drug screening experiment which facilitates retrospective data analysis when a result is not obtained from the initial target library search.
Confirmation testing using Xevo TQ Absolute or Xevo TQ-S micro Mass Spectrometers provided retention time and MRM semi-quantitative data for the compounds identified. Both mass spectrometers provided excellent levels of sensitivity to detect and quantify the compounds. For the counterfeit M30 pill analyzed, xylazine was detected and quantified at a concentration of 913 ng/mL.
References
- https://www.dea.gov/sites/default/files/2022-12/The%20Growing%20Threat%20of%20Xylazine%20and%20its%20Mixture%20with%20Illicit%20Drugs.pdf Accessed 31 May 2023.
- Michael Wakefield and Erik Todd. Seized Drug Screening using the ACQUITY RDa Detector. Waters Application Note. 720007097. December 2020.
720008060, October 2023