The Benefits of ACQUITY™ Premier UPLC for Multi-Mycotoxin Methods
Abstract
Multi-mycotoxin methods typically present challenges, such as uneven response for different compounds, carryover in LC systems, and matrix effects. These factors can affect validation experiments and significantly impact the performance of the method. To address these challenges, Waters has developed the ACQUITY Premier System and analytical columns, which incorporate the MaxPeak™ High Performance Surfaces (HPS) Technology. In this work we successfully transferred a previously developed multi-mycotoxin LC-MS/MS method on the new ACQUITY Premier System, and we observed a significant reduction of carryover for fumonisins by almost 80% compared to a conventional UPLC. In addition, very good method performance was achieved, in terms of linearity, precision, peak shape, and retention time stability.
Benefits
- The ACQUITY Premier System and column effectively reduce carryover of fumonisins compared to conventional UHPLC systems
- On the ACQUITY Premier System, the addition of metal chelators in the mobile phase or washing solutions was not necessary
- Analytical throughput was improved on the ACQUITY Premier System as the number of washing cycles can be reduced
- Very good linearity, precision, peak shape, and retention time reproducibility were obtained, which allowed to meet SANTE/12089/2016 performance guidelines
Introduction
We previously developed a UPLC-MS/MS method for regulated mycotoxins based on a dilute-and-shoot sample preparation procedure, the method was then expanded to determine a wider number of natural toxins.1,2 Multi-mycotoxin methods typically present challenges. The chemical diversity of toxin compounds gives rise to uneven response factors as well as carryover issues in LC systems for some classes of compounds. Also, the complexity of various food and feed products often leads to significant matrix effects. These challenges can affect validation experiments and significantly impact the performance of the method.
Incorporating an effective clean-up step into the sample preparation protocol can reduce the amount of unwanted co-extractives introduced into the UPLC-MS/MS system, thus mitigating the impact of matrix effects and enhancing the robustness of the method.3 Alternatively, matrix effects can be mitigated by using a high-sensitivity tandem quadrupole MS, such as the Xevo™ TQ-Absolute mass spectrometer allowing higher sample dilution factors (up to x 100) to be applied.
When using modern LC modules, a small amount of sample or analyte can often get trapped somewhere in the wetted flow path, resulting in unexpected or extra (ghost) peaks in consecutive chromatograms. This is generally referred to as “carryover” and can be one of the most frustrating problems in liquid chromatography and can hit values from 3 to 20% (percentage area in a blank injection following a high-level standard/sample injection). In different work, we studied the carryover of fumonisins B2 (FB2), which was used as a case-study to compare various strategies to minimize the amount of analyte remaining in the flow path following the use of different injector systems and configurations.4 The chemical properties of FB2 and similar compounds can lead to poor reproducibility and potential false-positive results. For these reasons, it is important to reduce carryover to an acceptable level. Typically, this is done by adding chelators like ethylenediaminetetraacetic acid (EDTA) or citric acid to the mobile phase or sample diluent. However, chelators can cause ion suppression and may be difficult to remove from LC systems.
To address these challenges, Waters has developed the ACQUITY Premier System and analytical columns, which incorporate the MaxPeak HPS technology.5 HPS is composed of a highly crosslinked layer containing enthylene-bridged siloxane groups, and it provides a highly effective barrier that mitigates undesired interactions with the metal surfaces in the flow path.
In this work, the previously developed multi-toxin method was transferred to the new ACQUITY Premier UPLC System and a comparison study was performed to assess whether the MaxPeak HPS technology can reduce carryover of those mycotoxins that are known to be partially adsorbed on the stainless-steel surfaces due to metal chelation. System sensitivity, retention time reproducibility, peak shape, and overall method performance has been also evaluated, in accordance to SANTE guidelines.6
Experimental
Sample and Standard Preparation
Wheat, oat, and wholemeal flour samples were extracted following a procedure based on previous work.1,2 Briefly, 5.0 g of homogenized sample were placed in a 50 mL plastic centrifuge tube and extracted with 20 mL 79:20:1 MeCN:H2O:acetic acid (v/v/v), on an automated Vortex for ten minutes. After centrifugation for six minutes at >5000 g, a portion of the supernatant was filtered through a 13 mm d, 1.2 µm glass fiber syringe filter, and diluted 1:5 with water directly into a LC vial (i.e. 100 µL of extract were mixed with 400 µL of H2O), resulting in an overall dilution factor of 20. Prior to LC-MS/MS analysis, 10 µL internal standard mixture containing 13C-labelled internal standards were added to each vial.
Solvent calibration curves containing the target analytes were prepared by serial dilutions of a Stock Mix solution, maintaining a solvent composition of H2O:MeCN 95:5 (v/v). 10 µL internal standard solution was added to 500 µL of each calibration standard.
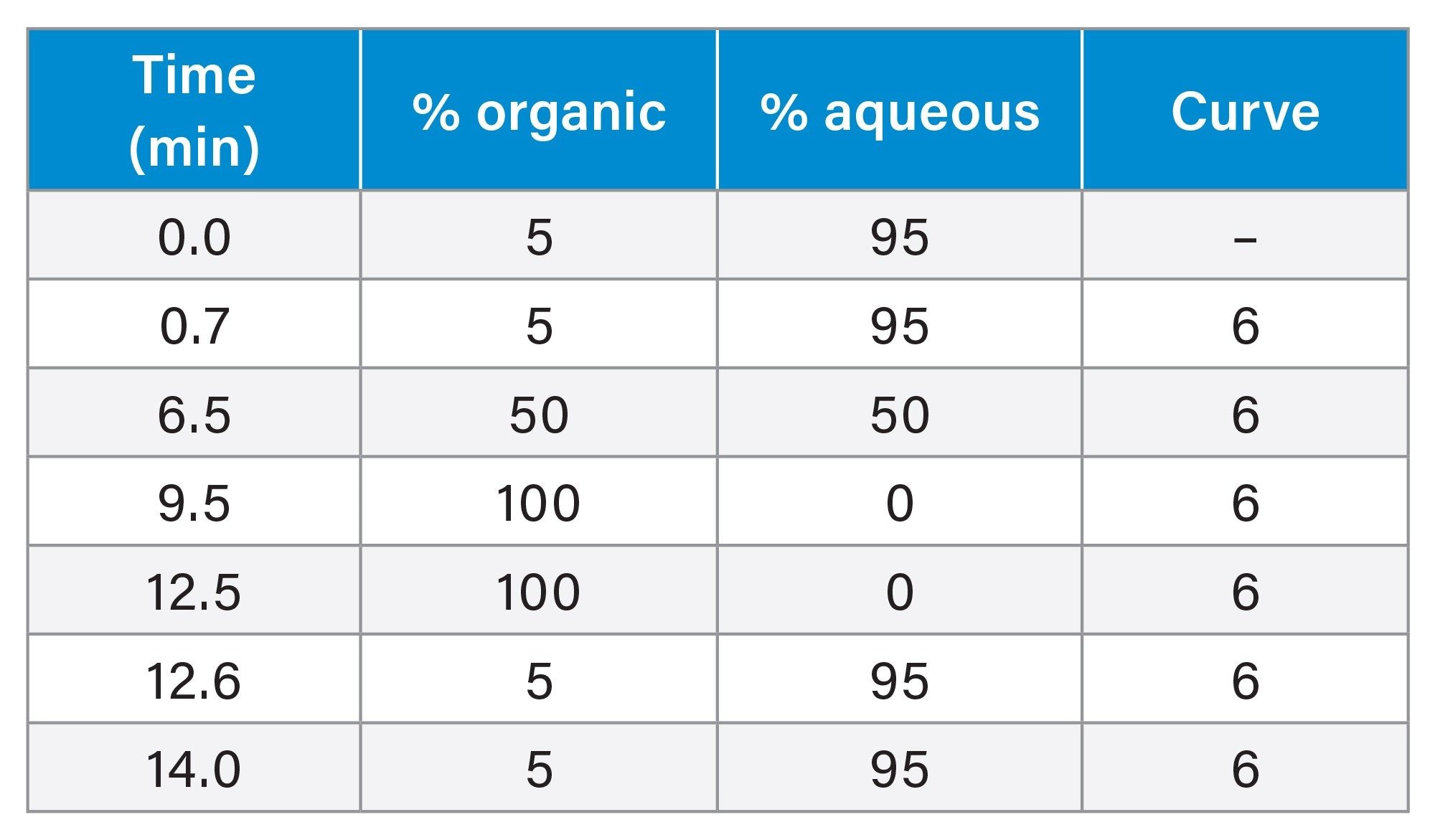
Liquid Chromatography Conditions
|
Chromatographic systems: |
Standard ACQUITY UPLC I-Class PLUS and ACQUITY Premier UPLC (with binary solvent manager) |
|
Autosampler and injector: |
Flow Through Needle injector (FTN) with 15-µL needle size and 50-µL HSP extension loop (p/n: 700012825) installed between port 6 of injection valve and APH. |
|
Columns: |
Standard system: ACQUITY UPLC BEH C18 1.7 µm (p/n: 186002352) Premier system: ACQUITY Premier BEH C18 1.7 µm (p/n: 186009453) |
|
Mobile phase: |
Aqueous: 1 mM ammonium acetate in water +0.3% acetic acid +0.1% formic acid (v/v) Organic: methanol +0.3% acetic acid +0.1% formic acid (v/v) |
|
Needle wash solvent: |
water: methanol: acetonitrile: isopropanol: acetone 20:20:20:20:20 + 0.1% formic acid (volumetrically) |
|
Seal wash solvent: |
water: methanol 80:20 (v/v) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
15 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.40 mL/min |

Mass Spectrometry Conditions
|
Mass spectrometry system: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI+/- (polarity switching) |
|
Acquisition mode: |
Multiple Reaction Monitoring (MRM) |
|
Capillary voltage: |
+0.75/-0.3 kV |
|
Cone gas flow: |
50 L/Hr |
|
Desolvation temperature: |
600 °C |
|
Desolvation gas flow: |
1100 L/Hr |
|
Source temperature: |
150 °C |
|
Resolution: |
MS1 Unit, MS2 Unit |
|
Data acquisition and processing: |
waters_connect™ for quantitation (v. 1.1) |
Note: A comprehensive list of optimized MRM transitions, cone voltages, and collision energies are reported in the previous work.7
Results and Discussion
Carryover Mitigation
Tamura and coworkers verified experimentally that the dissociated carboxyl groups in the fumonisins can form chelates with metals in the sample flow path resulting in analyte carryover in the subsequent injections.8
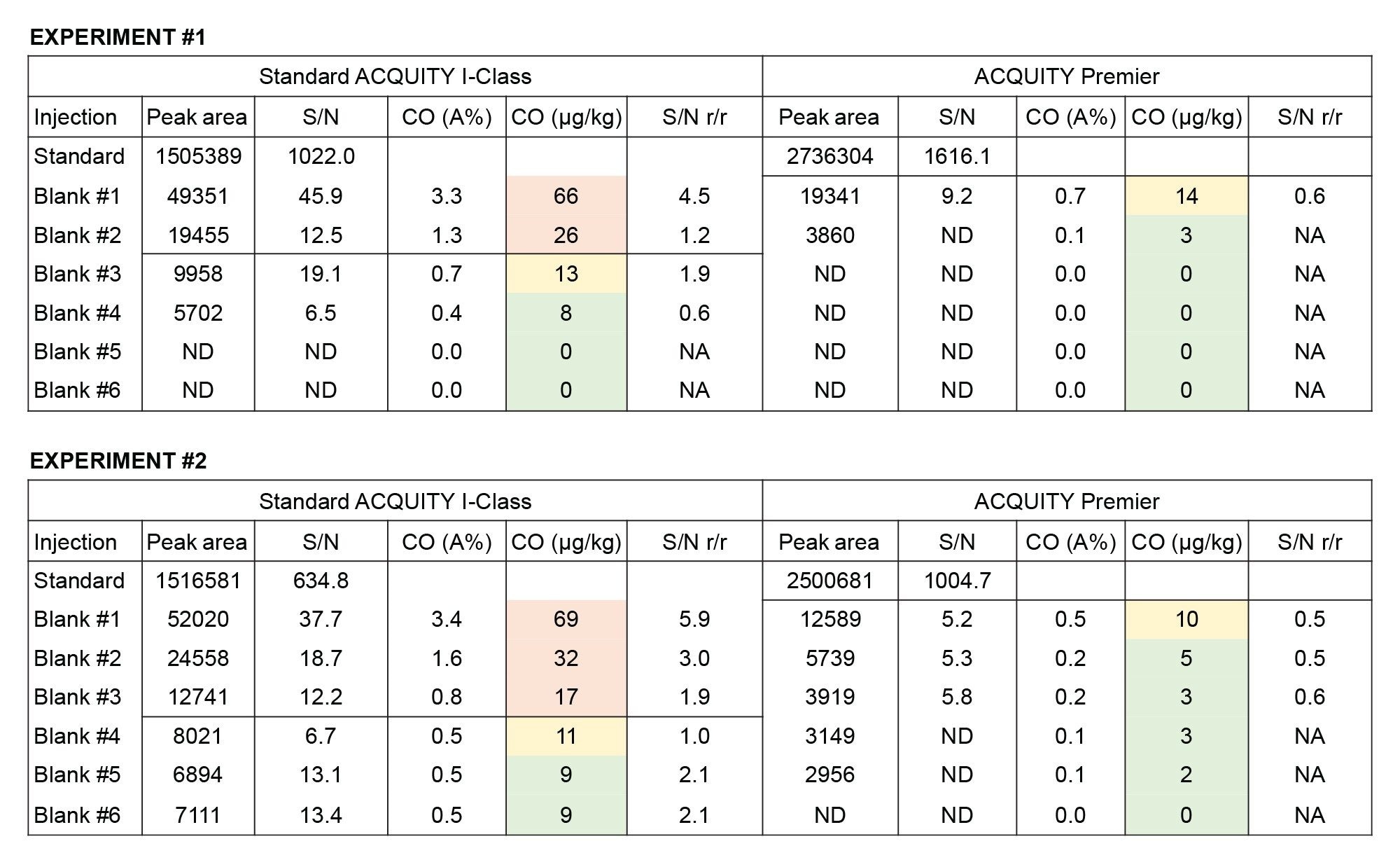
An experiment has been performed to assess the carryover of mycotoxins in the two systems – a sequence consisting of injecting 3x solvent blanks, 1x top-level calibrant, 6x solvent blanks was acquired multiple times, and carryover was calculated as percentage area (CO [A%]), and signal-to-noise ratio/ratio (S/N r/r).4
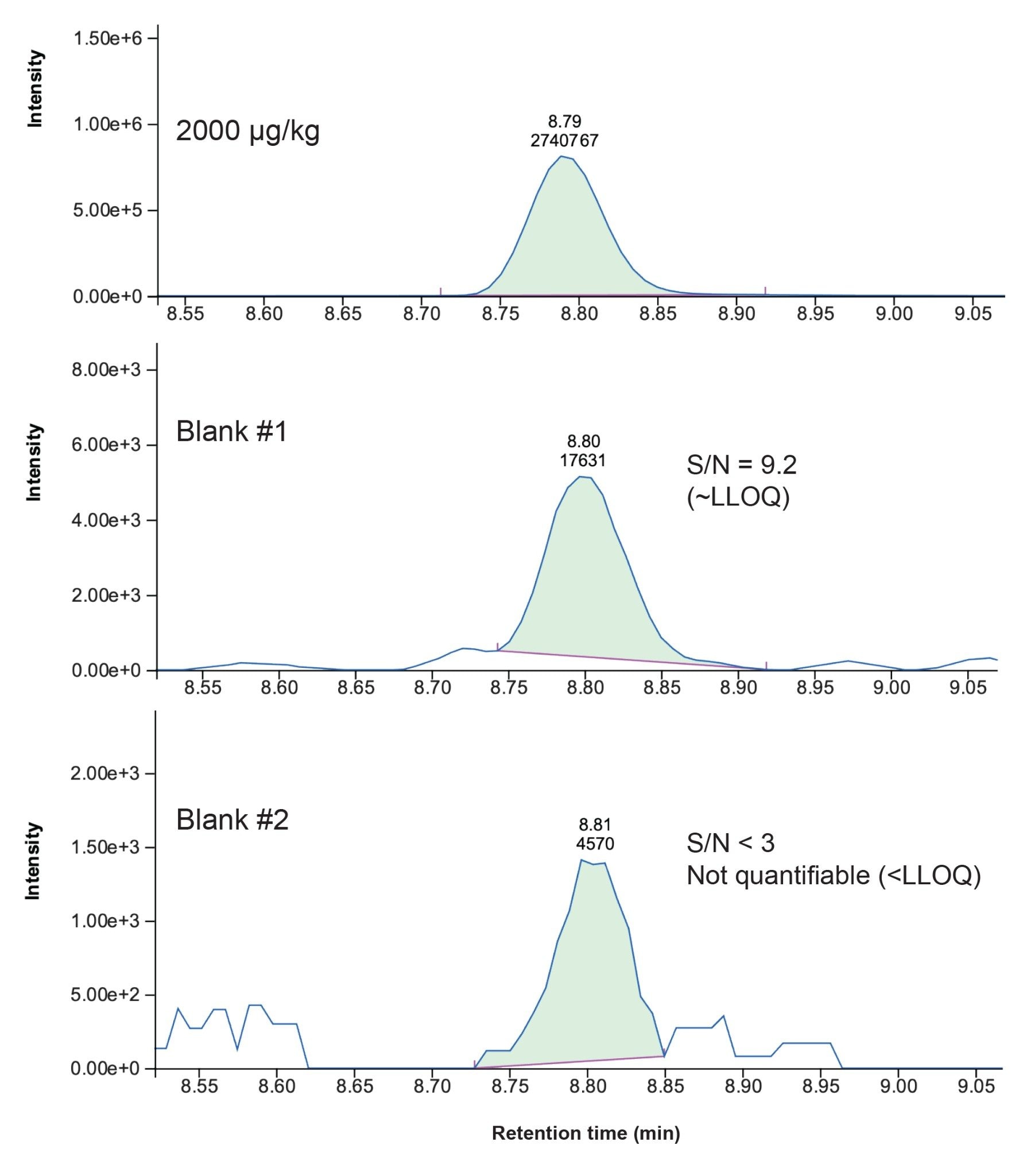
In Figure 2, the results of the experiment repeated twice on different days are reported. In this figure, blank injections where carryover is hitting values above the LLOQ are highlighted in red, in yellow are carryover values within 65% of the LLOQ, while in green values that are below the LLOQ (thus considered acceptable). It can be noted that on a standard ACQUITY I-Class, as well as on the majority of other vendors’ UHPLCs, it usually takes from two to three blank injections to bring the level of FB2 below the lower limit of quantification (LLOQ = 15 µg/kg) after injecting a highly concentrated standard or highly contaminated sample (in this case 2000 µg/kg of FB2). By contrast, on the ACQUITY Premier System, the response of FB2 in the first subsequent blank was comparable to or below the LLOQ, whilst the second blank showed no-quantifiable levels of FB2. As a result of this, it is possible to reduce the number and/or duration of washing cycles within the same sequences, thus improving the analytical throughput on the ACQUITY Premier. In addition, repeated experiments led to very similar values, which brings additional credibility to the results.
In Figure 3 it is shown a comparison of FB2 traces and peak areas for the top-level standard and the subsequent solvent blank injections on the ACQUITY Premier System.
With respect to fumonisins B1 (FB1), on the standard ACQUITY I-Class System, carryover values were equivalent to 30, 14, 8 µg/kg, and not-detectable in the subsequent blanks following a 2000 µg/kg standard injection. Whilst on the ACQUITY Premier System, the first subsequent blank already presented a carryover level < LLOQ and the second blank injection showed no detectable peaks.
From these results it is apparent that the use of ACQUITY Premier System is beneficial as it significantly reduces the carryover of mycotoxins thanks to the HPS technology, which acts as an efficient barrier that mitigates analyte adsorption on metal surfaces.
Overall Performance Comparison
Incurred cereal samples as well as samples spiked with known concentrations of 12 regulated mycotoxins were analyzed using both configurations by setting the analytical sequence to replicate a typical sequence in a routine food & feed testing laboratory (Figure 4). General method performance such as precision, sensitivity, linearity, and peak shape were evaluated on the two systems.

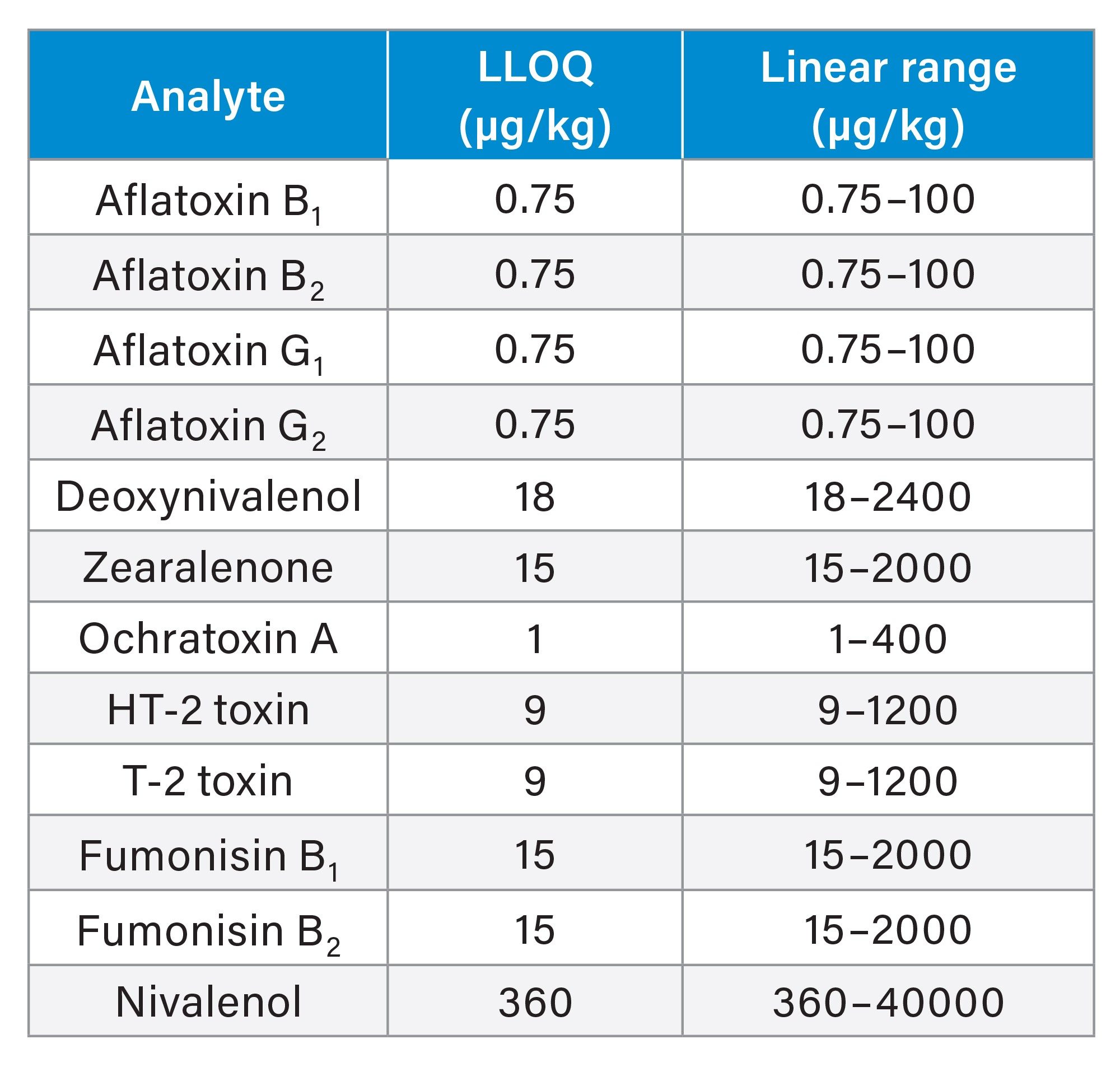
On both systems very good linearity was achieved for all mycotoxins, with coefficients of determinations (R2) > 0.99 and residuals well within ±20%. Lower limits of quantification (LLOQ) were comparable, although slightly higher peak areas and S/N ratios were observed on the ACQUITY Premier for some compounds. In Table 2, LLOQs and method linear range are reported, while in Figure 5 peak response and S/N ratio comparison for two representative mycotoxins at the LLOQ level are shown.
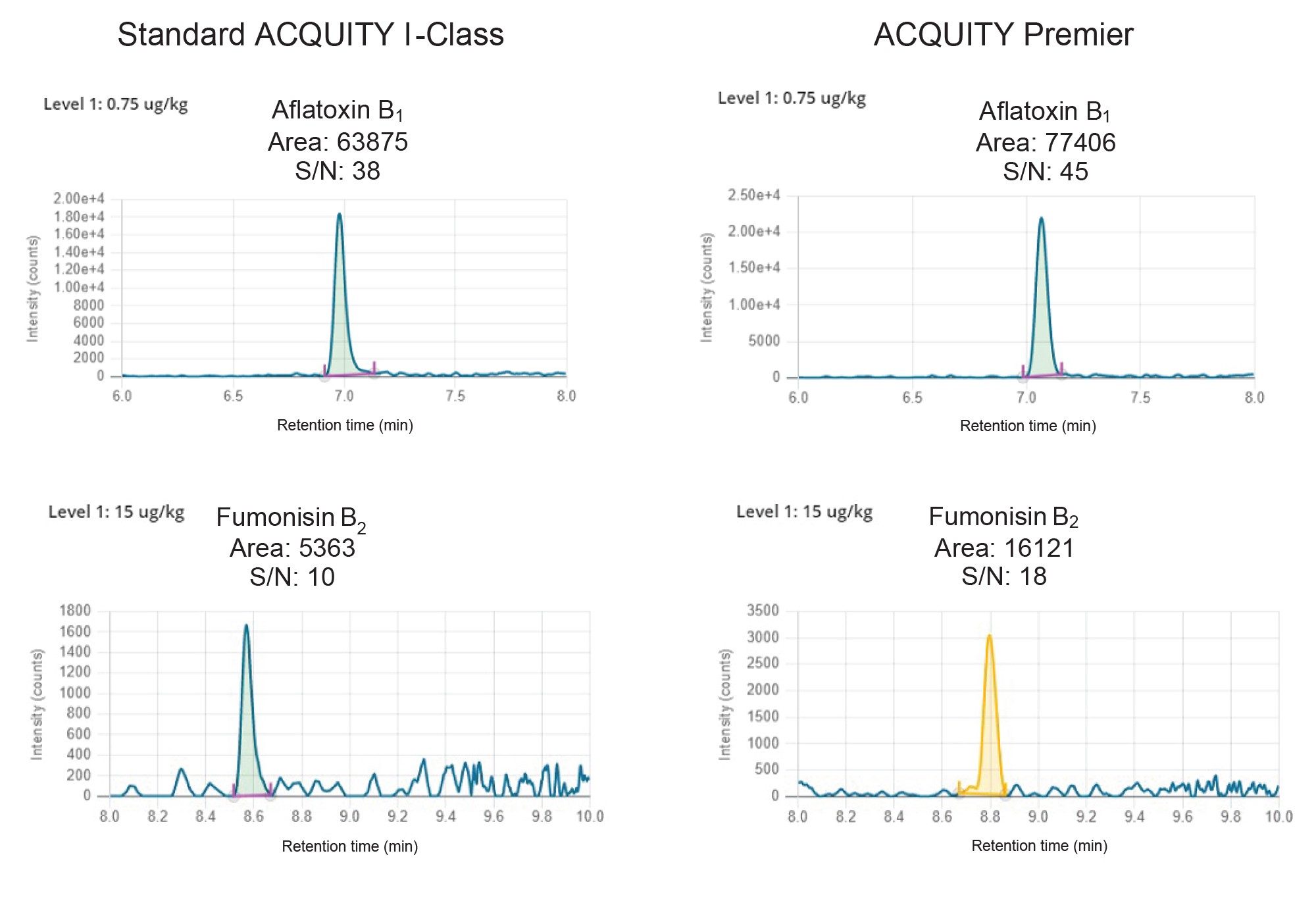
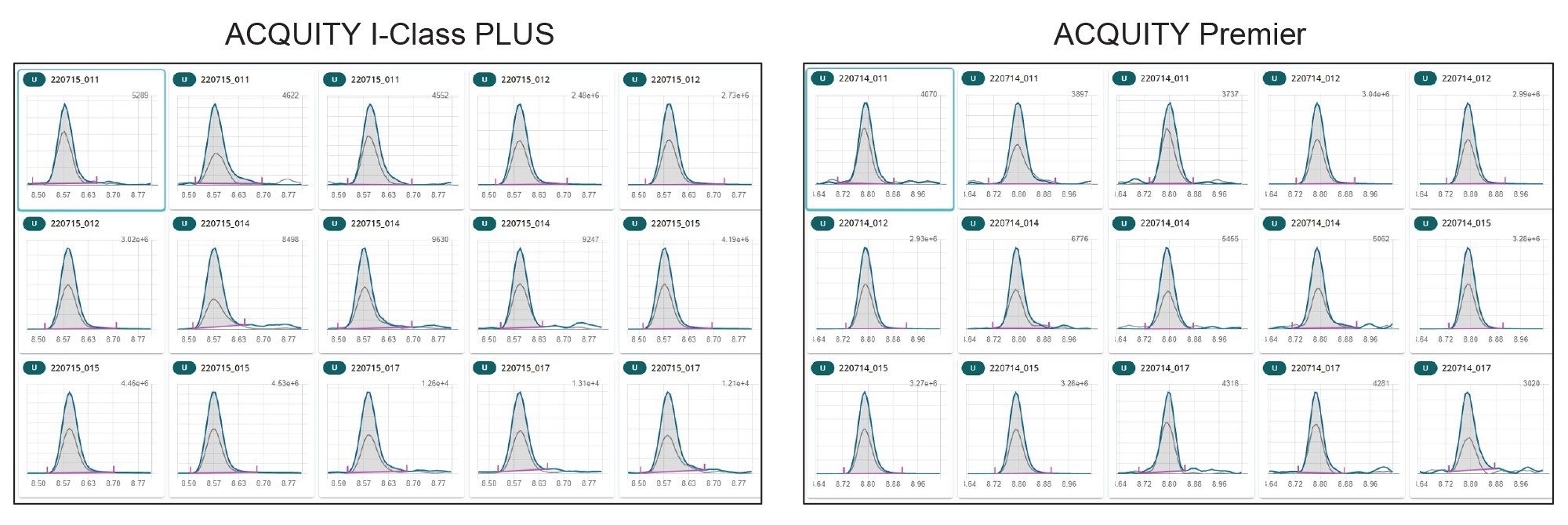
Relative standard deviations (RSD%) under repeatability conditions calculated over three replicates of incurred and spiked samples where below 10% for all analytes on both systems. Very good retention time reproducibility was achieved, with retention time deviations below 0.006 minute within the same sequence for all analytes. In addition, comparable peak shape was obtained across the two systems. As an example, in Figure 6 chromatographic traces of FB2 in 15 cereal samples are reported. It can be noted that on both systems Gaussian peaks with no tailing were obtained.
Conclusion
The previously developed multi-mycotoxins method with internally standardized calibration has been successfully transferred to the new ACQUITY Premier System with minimal modifications of the LC conditions. When running a typical routine analysis of food samples, including multiple replicates of different batches, equivalent method performance was obtained. Very good linearity, accuracy, precision, peak shape, and retention time reproducibility were observed across all analytes, as defined by SANTE guidelines.
The main benefit of the ACQUITY Premier System resides in the HPS technology, which enabled to significantly reduce the carryover of fumonisins by almost 80%. This allowed for the removal of metal chelators in the mobile phase and needle wash solutions, , and it also improved analytical throughput, as fewer washing cycles are needed within the same sequence.
References
- Dreolin N. and Stead S. LC-MS/MS Method Development and Validation for the Quantitative Determination of Regulated Mycotoxins in Cereal Grain Flours Using Simplified Sample Preparation Conditions on Xevo TQ-XS. Waters Application Note, 2019, 720006685.
- Dreolin N.; Foddy H.; Hird S.; Hancock P. and Jenkins T. Development of a Multi-Toxin UPLC-MS/MS Method for 50 Mycotoxins and Tropane Alkaloids in Cereal Commodities. Waters Application Note, 2021, 720007476.
- Dreolin N.; Foddy H.; Hird H.; Hancock P. and Jenkins T. Improve the robustness of an LC-MS/MS method for the determination of multiple mycotoxins in a range of food matrices. Waters White Paper, 2022, 720007521EN.
- Dreolin N. Carryover in UPLC methods: the case-study of fumonisins B2. Waters White Paper, 2020, 720006826en.
- Walter T. H.; Trudeau M.; Simeone J.; Rainville P.; Patel A. V.; Lauber M. A.; Kellet J.; DeLano J.; Brennan K.; Boissel K.; Birdsall R. and Berthelette K. Low adsorption UPLC system and columns based on MaxPeak High performance Surfaces: the ACQUITY Premier solution. Waters White Paper, 2021, 720007128en.
- SANTE/12089/2016. Guidance document on identification of mycotoxins in food and feed. Implemented by 01/01/2017.
- Dreolin N.; Stead S.; Hird S.; Jenkins T. Determination of Regulated and Emerging Mycotoxins in Cereals, Nuts, Figs, and Animal Feeds Using Pass-Through SPE and UPLC-MS/MS. Waters Application Note, 2021, 720007377.
- Tamura, M.; Matsumoto, K.; Watanabe, J.; Iida, J.; Nagatomi, Y.; Mochizuki, N. Minimization of carryover for high-throughput liquid chromatography with tandem mass spectrometry analysis of 14 mycotoxins in corn grits. Journal of Separation Science. (2014). DOI: 10.1002/jssc.201400099.
720007773, October 2022