For forensic toxicology use only.
In this application, a comprehensive comparison of sample preparation techniques including SPE, SLE, and LLE was conducted in plasma and urine, using a wide variety of compounds found in bioanalysis and forensic toxicology.
Oasis PRiME HLB also demonstrated superior recoveries and matrix effects for a variety of tested drugs without any additional method development. SLE and LLE required additional method development or multiple extraction protocols to achieve recoveries that were comparable to Oasis PRiME HLB for all of the tested analytes. The unique nature of Oasis PRiME HLB enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow. The μElution format enabled the direct injection of extracts without evaporation or reconstitution.
Solid-phase extraction (SPE) is a sample preparation technique by which compounds that are dissolved or suspended in a liquid matrix are extracted according to their physical and chemical properties. Reversed phase SPE sorbents can be either polymeric or silica based. In both cases, compounds are retained on the sorbent mainly by hydrophobic interactions. A washing step helps to remove matrix interferences. The analyte(s) can be eluted with an organic solvent, which disrupts the interaction of the analyte and the sorbent.1,2 Waters Oasis PRiME HLB is a novel reversed phase SPE sorbent developed to enable simpler and faster SPE protocols, while at the same time generating cleaner extracts than other sample preparation methods with a simple, generic three step protocol.
Liquid-liquid extraction (LLE) employs water-immiscible solvents to extract analytes from aqueous solutions. This is usually accomplished by shaking and collecting the solvent layer containing the analytes of interest.
Supported liquid extraction (SLE, aka, solid supported liquid extractionSSLE) is analogous to traditional liquid-liquid extraction (LLE) and utilizes the same water-immiscible solvent systems for analyte extraction from aqueous solutions. Instead of shaking the two immiscible phases together as in LLE, in SLE, the aqueous sample is immobilized on an inert support, and the organic phase flows through the supported matrix to extract the targeted analytes.3
In this application note, a comparison was performed between Oasis PRiME HLB SPE, SLE, and LLE in both plasma and urine matrices for bioanalysis and forensic toxicology. In plasma, 22 commonly analyzed pharmaceuticals, steroids, and drugs of abuse were extracted using the three aforementioned methods and the results were compared. In urine, 23 drugs of abuse representing opioids, stimulants, benzodiazepines, and synthetic cannabinoid metabolites were tested for forensic toxicology analysis.
Key areas of comparison were: procedure simplicity, analyte recoveries, and matrix effects (ME). The mechanisms behind these three techniques and how they affect their respective performances are discussed as well. Oasis PRiME SPE shows very high and consistent recoveries and excellent matrix effects across all of the tested analytes in both matrices. For SLE and LLE, lower recoveries were observed for polar basic analytes in urine samples and acidic analytes in plasma samples. The LLE and SLE methods were then optimized for these specific compounds and improvements in the recoveries of problematic analytes were successfully achieved, but only at the expense of other analytes. Only Oasis PRiME HLB was able to successfully extract all analytes from plasma and urine samples with a single method.
RCS-4 M10, RCS-4 M11, JWH-073 4-COOH, JWH-073 4-OH, and JWH-018 5-COOH were purchased from Cayman Chemical (Ann Arbor, MI). All other compounds and metabolites were purchased from Cerilliant (Round Rock, TX).
Individual stocks (1 mg/mL) were prepared in methanol, DMSO, or 50:50 DMSO:methanol. A combined stock solution of all compounds (5 μg/mL) was prepared in methanol, except naproxen, which was at 50 μg/mL. Working solutions were prepared daily by spiking standards into matrices (plasma and urine) and performing serial dilutions to achieve the desired concentrations.
In plasma, 22 drugs were analyzed including acids (naproxen), bases (most analytes), and neutrals (phenacetin, 17 α-OH progesterone) used in a variety of application areas. In urine, 23 drugs of abuse representing opioids, stimulants, benzodiazepines, and synthetic cannabinoid metabolites were tested.
|
LC system: |
ACQUITY UPLC I-Class, (FL) |
|
Column: |
CORTECS C18, 1.6 μm, 2.1 × 100 mm |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Flow rate: |
0.6 mL/min |
|
Gradient: |
See Table 1 |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Strong needle wash: |
70/30 ACN/water with 2% formic acid |
|
Weak needle wash: |
5/95 ACN/water with 1% formic acid |
|
Injection mode: |
Partial loop with needle overfill |
|
Injection volume: |
2–5 μL |
|
Time (min) |
Profile %A |
Profile %B |
Curve |
|---|---|---|---|
|
0 |
95 |
5 |
|
|
2 |
75 |
25 |
6 |
|
6 |
50 |
50 |
6 |
|
6.1 |
30 |
70 |
6 |
|
7 |
5 |
95 |
6 |
|
7.5 |
95 |
5 |
6 |
|
9 |
95 |
5 |
6 |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
3.0 kV |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
150 L/Hr |
|
Desolvation gas flow: |
1000 L/Hr |
|
MRM transition monitored: |
See Table 2 |
In this evaluation, the protocol used with Oasis PRiME HLB was the generic 3-step load-wash-elute protocol. Depending on the matrix, either 400 μL of plasma diluted 1:1 with 4% H3PO4 or 400 μL hydrolyzed urine diluted 1:1 with 4% H3PO4 was directly loaded onto an Oasis PRiME HLB μElution Plate. No conditioning or equilibration was needed or performed for either matrix. The samples were then washed with 2 × 200 μL 5% MeOH and eluted with 2 × 25 μL 90:10 ACN:MeOH. The eluate was then diluted with 100 μL water, vortexed, and directly injected into the LC-MS system without evaporation or reconstitution.
For SLE, there are multiple dilution buffers (to dilute the biological sample for loading) and extraction solvents suggested depending on the analytes of interest. Since the aim of this work was to compare one single method targeting all compounds, we evaluated protocols with the highest likelihood of success. The protocols selected for this evaluation were designed for neutral and basic analytes as they are predominant in the mixture. For plasma samples, 400 μL diluted plasma (200 μL rat plasma + 200 μL water) was loaded into an SLE plate (obtained from a competitor, rigidly designed for 400 μL sample load). Loading was initiated by applying gentle vacuum (~ 3 psi) for 2–5 seconds and waiting 5 minutes for the sample to completely absorb onto the support matrix. To begin the extraction of the analytes, 800 μL of extraction solvent (MTBE: Methyl tert-butyl ether) was then applied and allowed to flow over the matrix for 5 minutes under gravity. Vacuum (10 psi) was applied again for 10–30 seconds to complete the elution. The extraction steps were then repeated by adding another 800 μL of MTBE. To ensure compatibility with LC-MS analysis and concentrate the analytes, the extract was evaporated to dryness under N2 gas flow at 40 °C and then reconstituted in 200 μL of 30% acetonitrile (ACN). For urine samples, two similar pretreatment protocols were used. 200 μL hydrolyzed urine was diluted 1:1 with either water or 0.5 M NH4OH. Samples were then loaded onto the SLE plate and processed as described above for plasma samples.
For LLE, the experiments were performed using single 2 mL centrifuge tubes. As LLE and SLE share a similar mechanism, similar protocols were applied. 1000 μL MTBE was added to either 400 diluted plasma or hydrolyzed urine for the LLE experiments. As with SLE, plasma samples were diluted with 200 μL water. 200 μL hydrolyzed urine samples were diluted with either 200 μL water or 200 μL of 0.5 M NH4OH. The samples were then vortexed for 5 min and centrifuged for 5 min at 11000 rcf. The top layer was transferred to a collection plate and evaporated to dryness under N2 gas flow at 40 °C and reconstituted in 200 μL of 30% acetonitrile (ACN).
Urine hydrolysis for all samples/techniques: 200 μL of spiked urine was mixed with 160 μL of water and 40 μL of β-glucuronidase enzyme (Roche, E. coli) at room temperature for 30 minutes to simulate enzymatic hydrolysis.
Analyte recovery was calculated according to the following equation:
% Recovery = (Area A/ Area B) x 100%
Where A equals the peak area of an extracted sample and B equals the peak area of an extracted matrix sample in which the compounds were added post-extraction.
Matrix effects were calculated according to the following equation: Matric Effects = ((Peak area in presence of matrix/Peak area in the absence of matric)-1) x 100%
The peak area in the presence of matrix refers to the peak area of an extracted matrix sample in which the compounds were added post-extraction. The peak area in the absence of matrix refers to analytes in a neat solvent solution.
A representative chromatogram of all compounds from a 20 ng/mL extracted plasma sample is shown in Figure 1. The urinary chromatography is shown in Figure 2. Using a CORTECS UPLC C18 Column (90Å, 1.6 μm, 2.1 x 100 mm), all analytes were analyzed within 6.5 minutes. Peak shape was excellent for all compounds, with no significant tailing or asymmetries, and all peak widths were under 3 seconds at 5% of baseline. All potentially interfering compounds such as methamphetamine and phentermine, which share an MRM transition (150>91) were baseline separated.
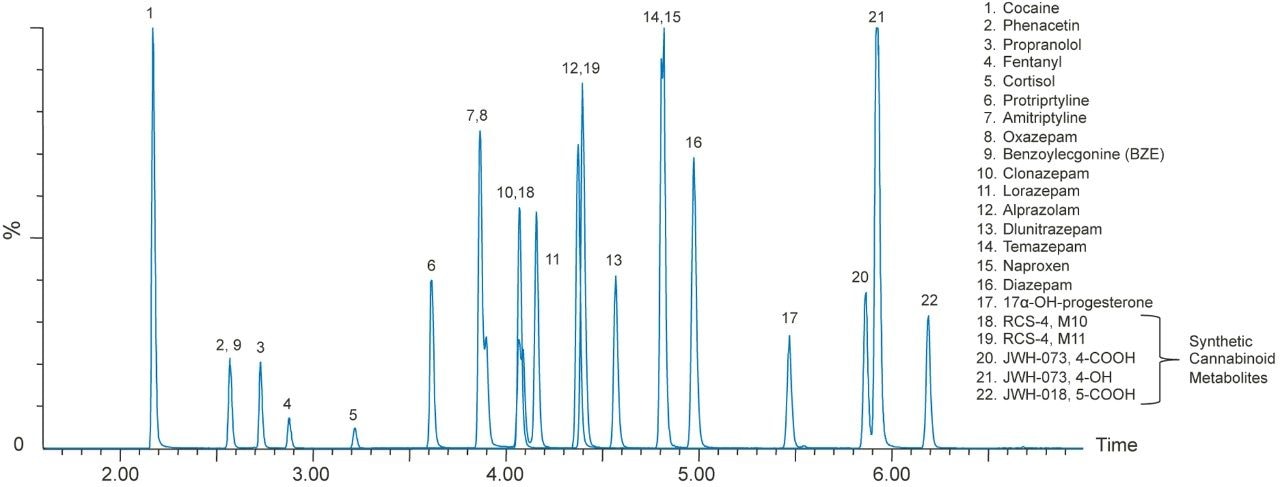
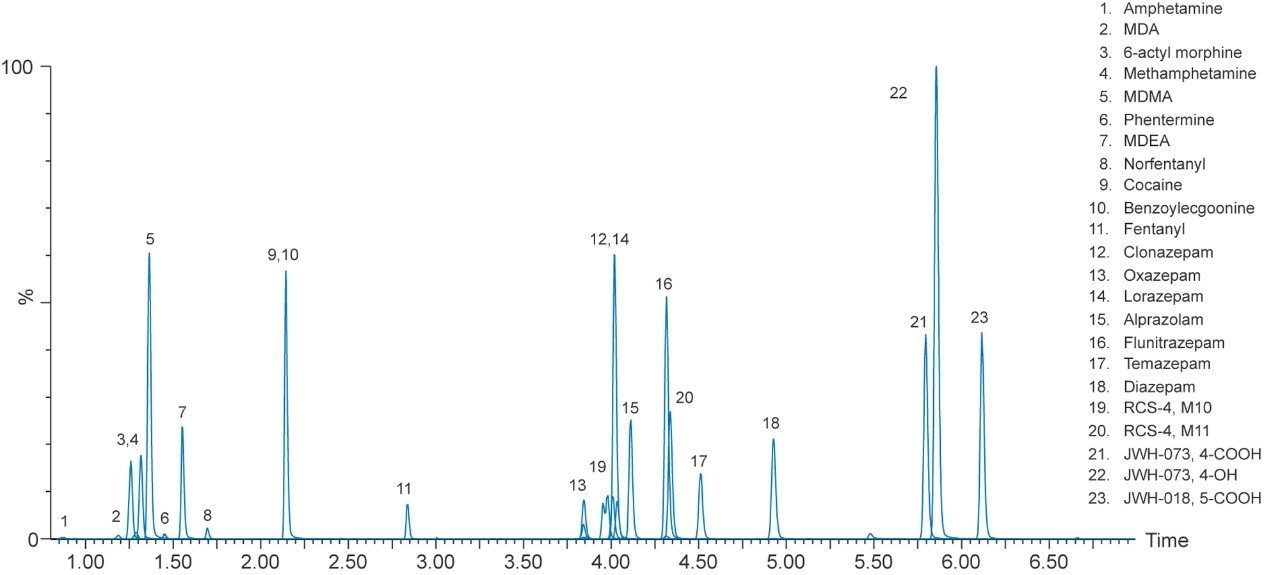
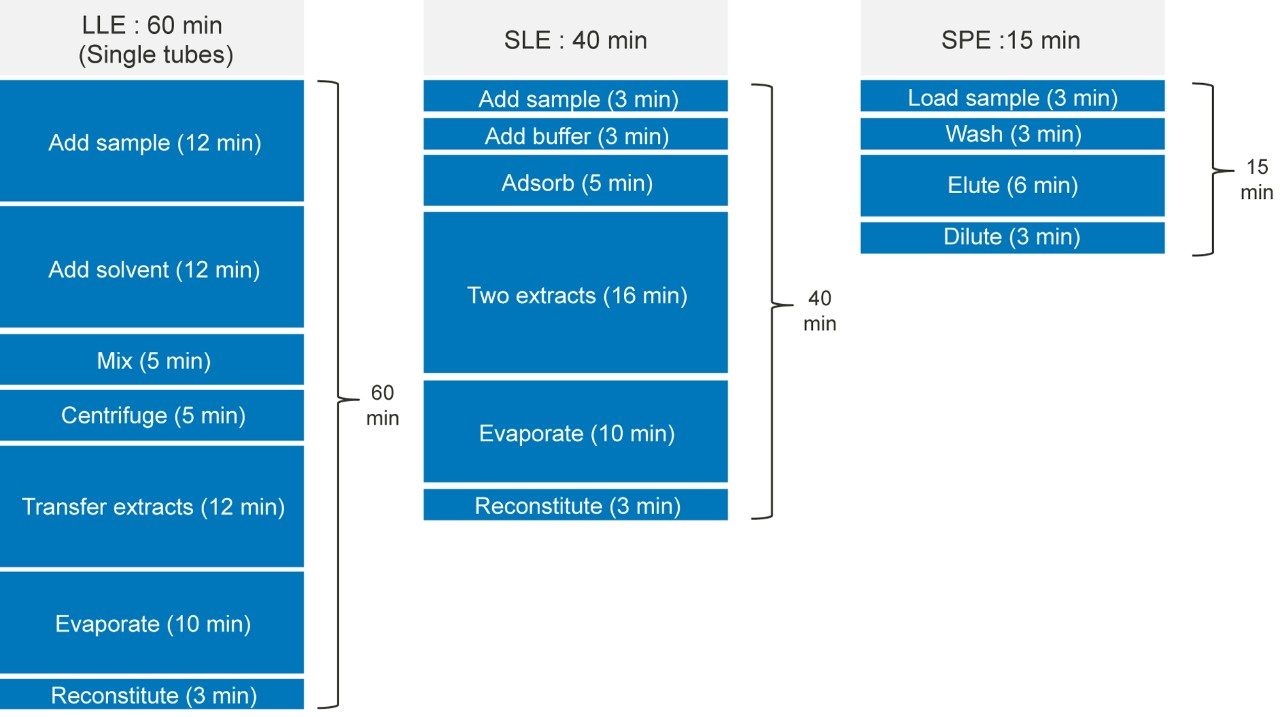
Figure 3 details the extraction protocols and processing time for SPE, SLE, and LLE. The total time required to prepare 96 plasma samples is 15 minutes for Oasis PRiME HLB, 40 minutes for SLE, and 60 minutes for LLE. Oasis PRiME HLB uses a simple, generic three step SPE technique that removes salt, proteins, and phospholipids without the need for evaporation and reconstitution (in the μElution format), whereas SLE and LLE require method development with different sample pretreatment or extraction solvents for different classes of analytes. SLE requires a 5 minute waiting time after loading to allow the sample to fully adsorb onto the support matrix. In addition, an additional 5 minute waiting time is required after the extraction solvent is applied to allow the analytes to interact with the extraction solvent. Since a water-immiscible solvent is used in extraction step, evaporation and reconstitution are required for LC-MS analysis. In addition, the initiation of the flow in the SLE sample loading step, which is accomplished by applying very gentle vacuum (~3 psi) for 2–5 seconds, is very subtle and takes time and practice to perfect. If the initiation time is too short (shorter than 2–5 seconds) or the pressure is too low, the aqueous sample won’t be able to successfully immobilize to the sorbent. If the time is too long or the pressure is too high, the plasma sample will directly elute and result in a cloudy elution solution and higher matrix factors. In SLE and LLE, the use of harsh water-immiscible extraction solvents may also extract impurities from the frits and plates, contaminating the extraction solution. Extraction solvents such as MTBE also have a negative impact on both operators’ health and the environment.
With the simple three step SPE protocol, Oasis PRiME HLB demonstrates excellent and consistent recoveries across all the tested analytes (Figure 4A) with an average % recovery of 98±8%. All tested recoveries were within 75–110%. SLE showed good recoveries for neutral and basic drugs, but poor recoveries for acidic analytes such as naproxen and the COOH metabolite of the synthetic cannabinoid, JWH-073. Average recoveries were 89±7%. All analyte recoveries for LLE were lower than 80% with an average recovery at 70 ± 10%. Only one extraction was performed during the experiment, which may have resulted in decreased extraction efficiency. A second extraction may have increased recovery, but would also have increased processing time. Previous work has also indicated that additional extractions can contribute to increased matrix effects. For SLE and LLE, the extraction method was selected for neutral and basic analytes. Acidic analytes such as naproxen, JWH-073, 4-COOH, and JWH-018, 5-COOH were not recovered well at all (less than 30% recovery). Further method development or a separate protocol would be required for SLE or LLE to improve acidic analyte recovery such as different sample pretreatment or buffering. However, this could adversely affect the recovery of the basic drugs. Under these conditions, only Oasis PRiME HLB was able to extract the full complement of basic, neutral, and acidic compounds with a single protocol.
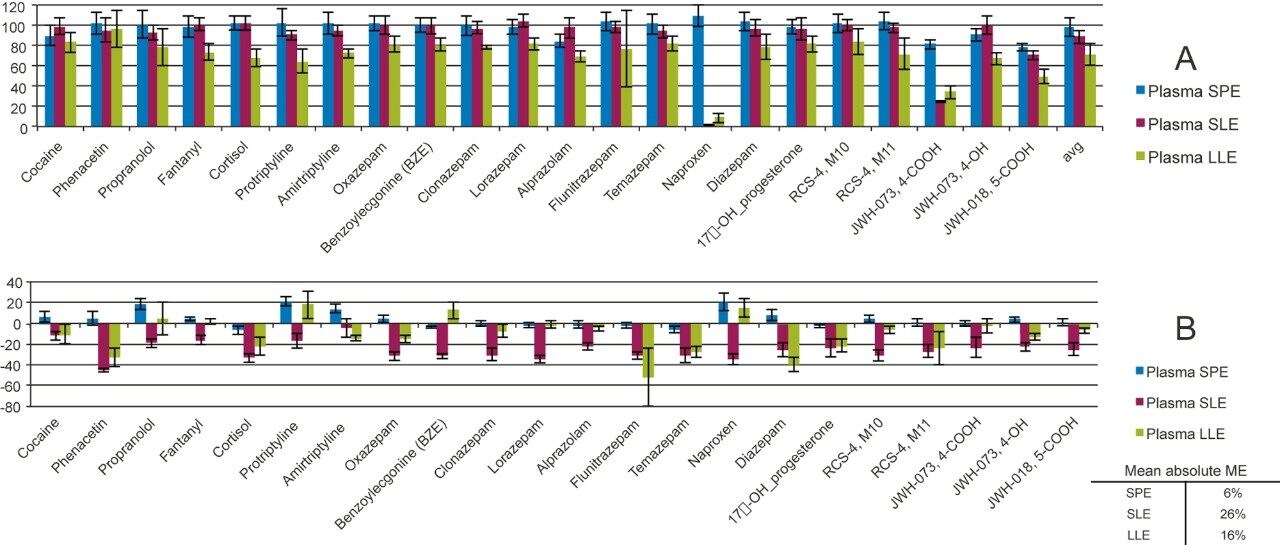
The overall matrix effects for Oasis PRiME HLB were lower than SLE or LLE (Figure 4B). All matrix effects for Oasis PRiME HLB were <20%, while 17/22 drugs from SLE and 7/22 drugs from LLE processing have MEs that are greater than 20%. The average magnitude of matrix effects for Oasis PRiME HLB was only 6%, while SLE was 26% and LLE was 16%. Furthermore, matrix effects for LLE were more variable. Matrix effect standard deviation values ranged from 1.4–8.8% for SPE, 1.9–10.3% for SLE and 2.6–28.3% for LLE. The three step protocol on Oasis PRiME HLB removed salts, proteins, and phospholipids and resulted in very clean final extracts with minimal matrix effects for all 22 different drugs, from several diverse classes. The higher matrix effects seen in SLE extracts may be a result of impurities extracted from the SLE sorbent as this wasn’t seen in LLE extracts where the same sample and extraction solvent were used. Alternatively, it could also be simply a result of the more efficient extraction seen with SLE vs. LLE. Since LLE appears to be more effective at extracting the analytes from urine, it may also extract other components that could contribute to ion suppression.
Overall, Oasis PRiME HLB demonstrated superior recovery and minimal matrix effects when the sample matrix contains a wide variety of compounds. In this case, this included acids (naproxen and the synthetic cannabinoid metabolites), bases (most drugs), and neutral compounds of varying polarities. SLE yielded acceptable recoveries for neutral and basic analytes, but with much higher matrix effects. LLE, due to its limited extraction efficiency, had lower recoveries (10–20% lower in recoveries compared to SPE and SLE). LLE also demonstrated higher variability in matrix effects, particularly for compounds such as flunitrazepam and propranolol. Using an SLE or LLE extraction, acidic analytes can’t be efficiently recovered with this single procedure, and additional method development would be required to improve acid recoveries.
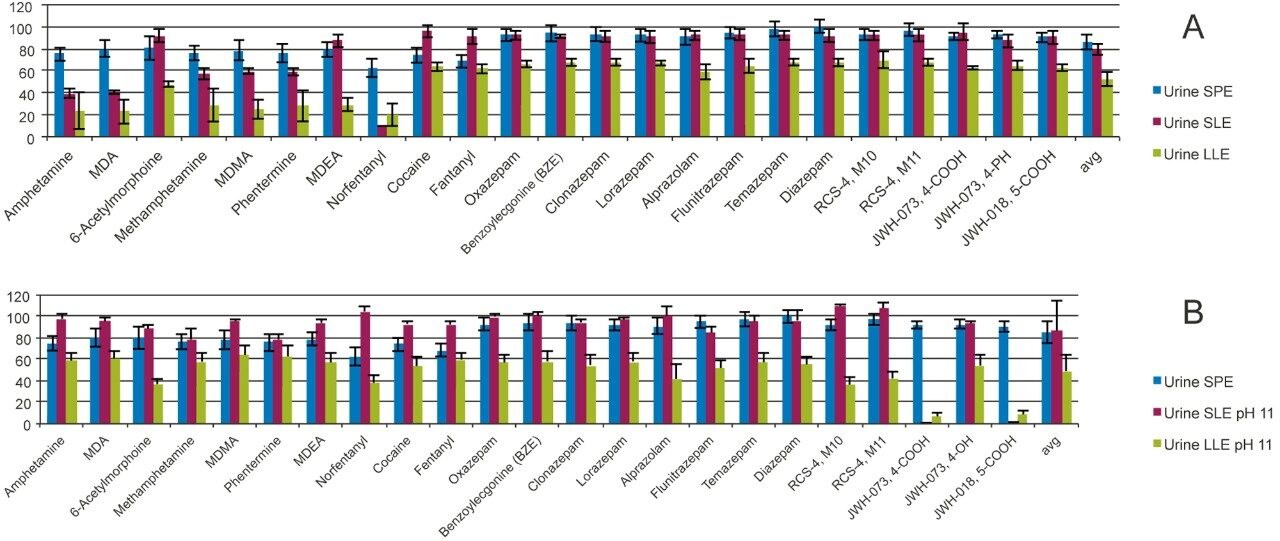
A wide panel of 23 drugs of abuse which included stimulants, opioids, benzodiazepines, benzoylecgonine (BZE), and synthetic cannabinoid metabolites was hydrolyzed and extracted with SPE, SLE, and LLE. As shown in Figure 5, high and consistent recoveries were obtained using the Oasis PRiME HLB generic 3-step protocol. Recoveries were >75% for 21/23 tested drugs and the overall average recovery was 86% ± 6.6%. Two extraction protocols for SLE and LLE extractions were performed as described in the materials and methods section. When samples were diluted with water (Figure 5A) SLE showed good recoveries for many drugs with the exception of the hydrophilic bases such as most of the amine stimulants and norfentanyl (the recoveries were lower than 60%). LLE exhibited a similar trend to SLE with much lower recoveries. When SLE and LLE extractions were performed after adjusting the pH of the urine samples to 11 with 0.5 M ammonium hydroxide, recoveries of the polar amines improved significantly (Figure 5B). However, this was at the expense of the more acidic compounds such as the carboxy metabolites of JWH-073 and JWH-018. Unlike Oasis PRiME HLB, a single protocol for SLE or LLE was unable to extract all of the analytes from the samples with acceptable recovery.
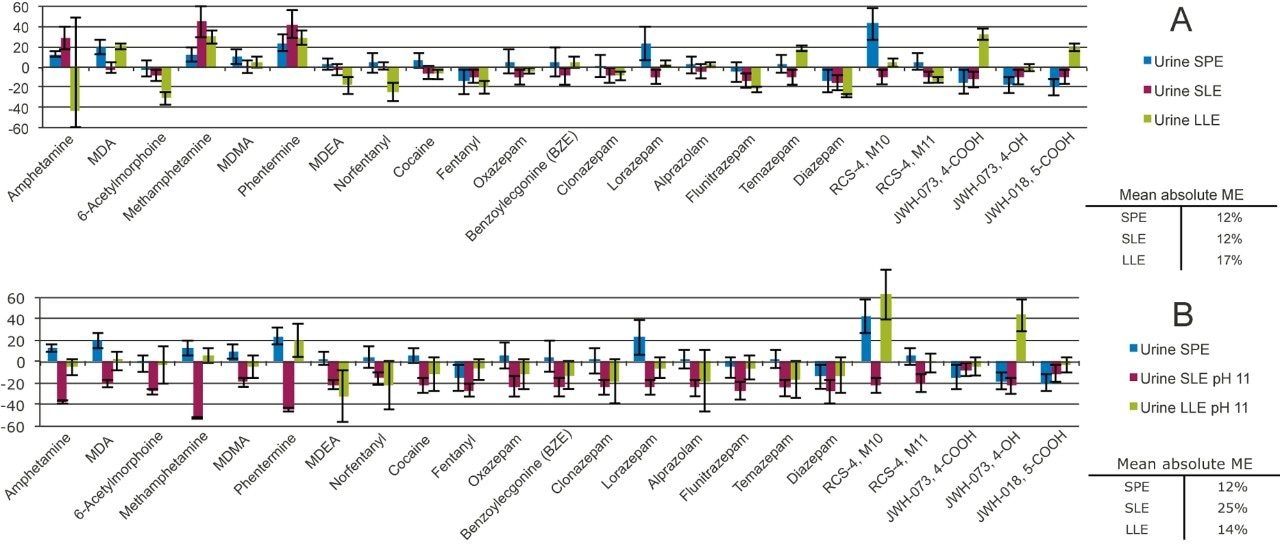
The matrix effects for Oasis PRiME HLB, SLE, and LLE are roughly comparable (Figure 6A). The absolute average of matrix effects for SPE, SLE, and LLE were 12, 12, and 17 respectively, all of which are acceptable. Matrix effects were within 20% for the majority of the compounds using any of the three extraction techniques. When the urine pH was adjusted with ammonium hydroxide (Figure 6B), matrix effects for SLE increased to an average of 25%, while those for LLE remained relatively low, with a mean absolute value of 14%.
In this application, a comprehensive comparison of sample preparation techniques including SPE, SLE, and LLE was conducted in plasma and urine, using a wide variety of compounds found in bioanalysis and forensic toxicology. Oasis PRiME HLB employed a simple, three step protocol in which reduced extraction time by 60% and 75% compared to SLE, and LLE, respectively, for forensic toxicology. Oasis PRiME HLB also demonstrated superior recoveries and matrix effects for a variety of tested drugs without any additional method development. SLE and LLE required additional method development or multiple extraction protocols to achieve recoveries that were comparable to Oasis PRiME HLB for all of the tested analytes. The unique nature of Oasis PRiME HLB enabled the elimination of conditioning and equilibration steps, simplifying the extraction procedure and speeding up the sample preparation workflow. The μElution format enabled the direct injection of extracts without evaporation or reconstitution.
720006060, August 2017