Application of Neutral Loss and Precursor Ion Scanning by Tandem Quadrupole Mass Spectrometry to Monitor for Drug Metabolites in in vivo and in vitro Samples
Abstract
Detection of drug metabolites in early discovery in vivo or in vitro studies, and clinical trials is essential to successful drug development, allowing metabolic soft spots to be detected in discovery, and new or toxic metabolites to be detected in the clinical phase. Constant neutral loss scanning and precursor ion scanning offers a simple and rapid approach to the detection of drug metabolites in biological fluids, (e.g., blood products, CSF, and urine) and in vitro incubations. Here we outline the application and benefits of constant neutral loss and precursor ion scanning experiments for drug metabolism studies.
Benefits
Rapid detection of drug metabolites and related metabolites in biological fluids. Expanding the versatility of tandem quadrupole MS.
Introduction
The detection, identification, and monitoring of in vitro and in vivo drug metabolites plays an essential role in drug discovery and development. The detection of drug metabolites in discovery studies, (in vitro or in vivo), allows metabolic soft-spots to be identified, route of elimination to be determined, and the pharmacokinetic characteristics of the molecule to be optimized.1,2 In preclinical development it is important to understand the effect of species and dose level on the metabolic profile of a compound and ensure sufficient metabolic coverage in toxicological species to support human clinical trials. In clinical trial studies candidate medicines are tested in real patient populations to evaluate efficacy, effect in special patient populations, and different ethnic groups. It is important to detect and monitor these samples for known metabolites from safety assessment studies and also detect unknown, potentially toxic metabolites.3
In these studies mass spectrometry coupled to liquid chromatography is the technology of choice for determining the exposure to the candidate drug and also to screen samples for known and unknown metabolites. Constant neutral loss and precursor ion scanning are two powerful and flexible modes of data acquisition which can be used to screen for drug metabolites based on a specific moiety, e.g. glutathione or the presence of a diagnostic fragment ion.4,5 In this application note we illustrate how these two acquisition modes can be used to screen for drug metabolites in biological fluids.
Experimental
Sample Description
Full details of the safety assessment study and sample preparation are given in Waters Corporation Application note Tandem Quadrupole Acquisition Modes in DMPK Studies.6
Method Conditions
The urine samples were analysed by the injection of a 2 µL aliquot of sample onto a 2.1 x 100 mm Cortecs™ C8 2.7 µm Column. The column was maintained at 40 °C and eluted with a linear reversed-phase gradient over 10 minutes at 600 µL/min using aqueous 0.1% formic acid as mobile phase solvent A and 0.1% formic acid in 95:5 (v/v) acetonitrile:water as mobile phase B. The column effluent was monitored by positive ion ESi mass spectrometry operating in either: i) constant neutral loss or ii) precursor ion scanning mode.
LC Conditions
|
LC system: |
ACQUITY™ I Class UPLC™ |
|
Detection: |
Xevo™ TQ-XS |
|
Vials: |
Waters™ Total Recovery Vials (p/n: 186004631) |
|
Column(s): |
2.1 x 100 mm Cortecs C8 2.7 µm (p/n: 186010473) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
8 °C |
|
Injection volume: |
2 µL (urine) |
|
Flow rate: |
600 µL/min |
|
Mobile phase A: |
0.1% (v/v) aqueous formic acid |
|
Mobile phase B: |
95% acetonitrile, 5% waters, 0.1% (v/v) formic acid |
|
Gradient: |
See table below |
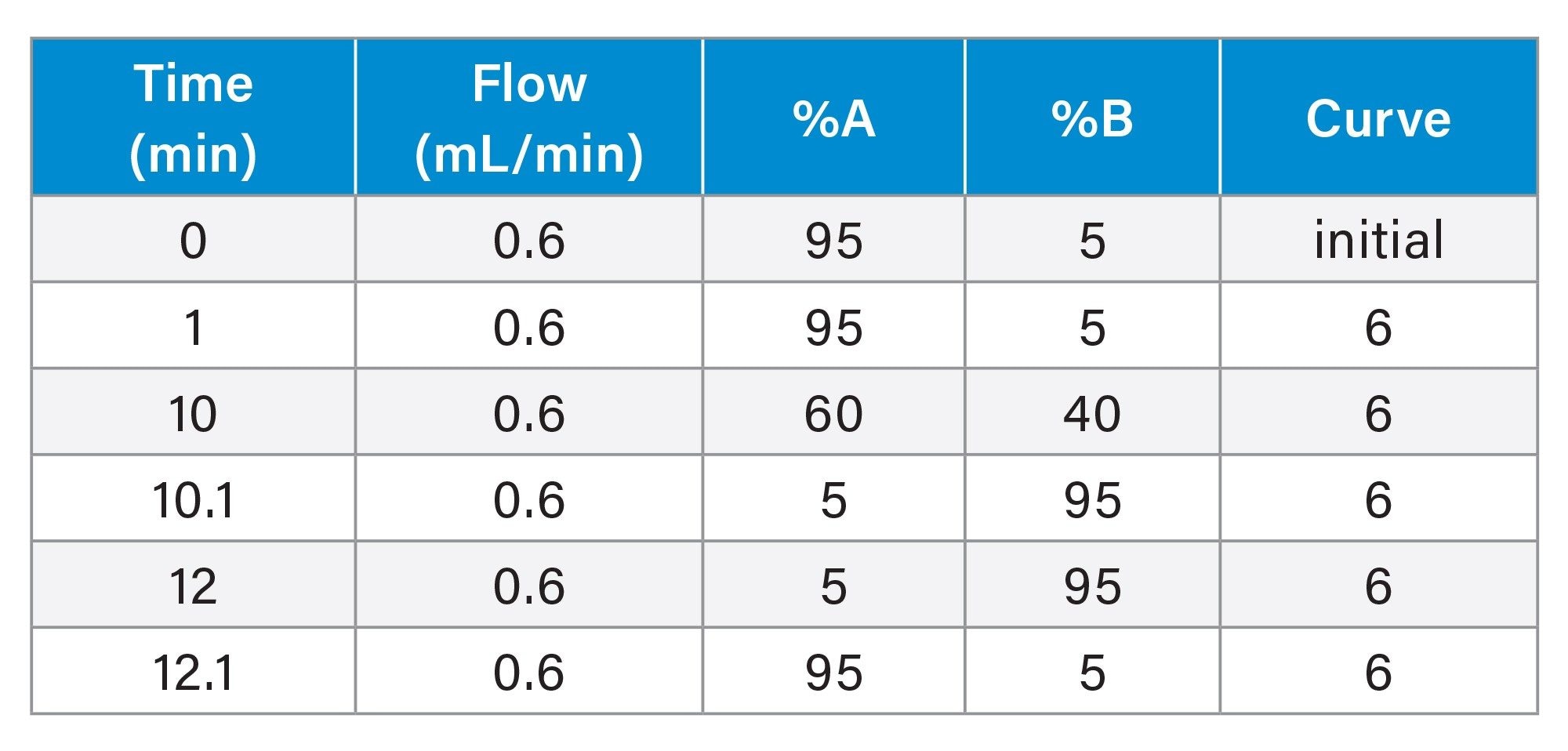
Gradient Table (urine and plasma screening analysis)
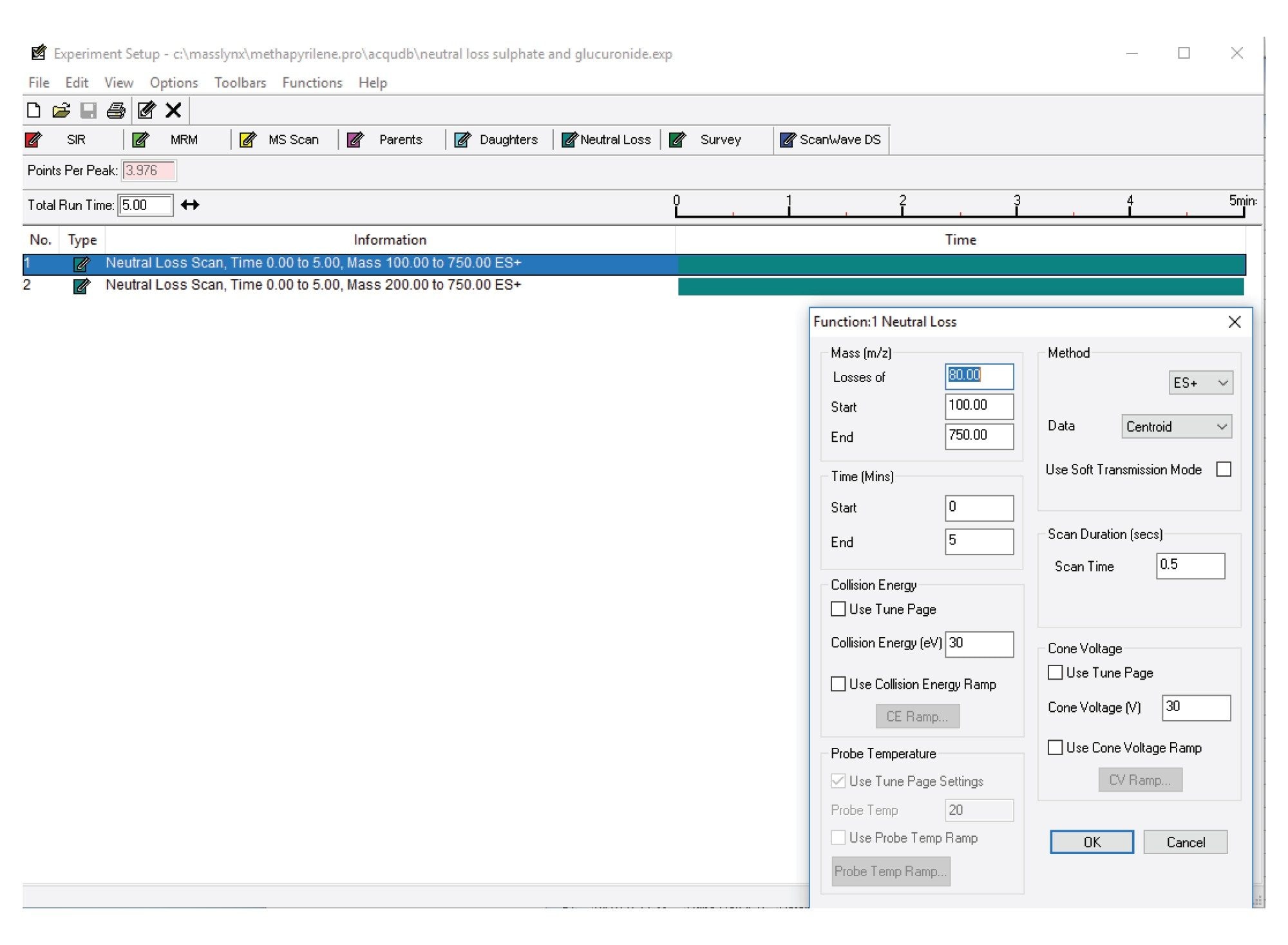
MS Conditions
|
MS system: |
Xevo TQ-XS |
|
Ionization mode: |
Positive Ion |
|
Acquisition range: |
m/z 50–800 |
|
Capillary voltage: |
2.0 Kv |
|
Collision energy: |
30 eV |
|
Cone voltage: |
30 V |
|
Neutral loss: |
80 Da and 176 Da |
|
Precursor ions: |
m/z=97, 119, 121 |
Data Management
|
Chromatography software: |
MassLynx™ Ver 4.2 |
|
MS software: |
MassLynx Ver 4.2 |
|
Informatics: |
MassLynx Ver 4.2 |
Results and Discussion
The rapid detection and characterization of drug metabolites in in vitro and in vivo studies is critical to efficient, safe drug discovery, and development. The detection of drug metabolites in incubation samples or biological fluids by LC-MS/MS is achieved by screening the samples using a variety of MS acquisition modes which are specific for either a diagnostic fragment in the parent compound or for a type of metabolic product such as sulphation or glucuronidation. Constant neutral loss scanning and precursor ion scanning are two modes of MS data acquisition which can provide either fragment or class specific data collection which can be used for metabolite profiling. Constant neutral loss and precursor ion scanning mass spectrometry data acquisition offer an information rich approach to analyte detection, (see Waters application note Tandem Quadrupole Acquisition Modes in DMPK Studies).6 Constant neutral loss scanning MS is based on the loss of a specific moiety and detected by the synchronized scanning of both quadrupoles. This mode of analysis can be used to detect common conjugation metabolites such as sulphate, glucuronide etc., as well as to detect potentially toxic types of metabolites such glutathione’s.4 Precursor ion scanning is an acquisition mode in which ions are scanned (over a pre-set mass range) in the first quadrupole, these ions are then fragmented in the collision cell, the final resolving quadrupole is set to allow one or more specific, diagnostic, fragment ions to be transmitted, and recorded at the detector.5 This acquisition mode allows researchers to use knowledge of the fragmentation pattern dosed compound to screen for drug related material in the biological samples.
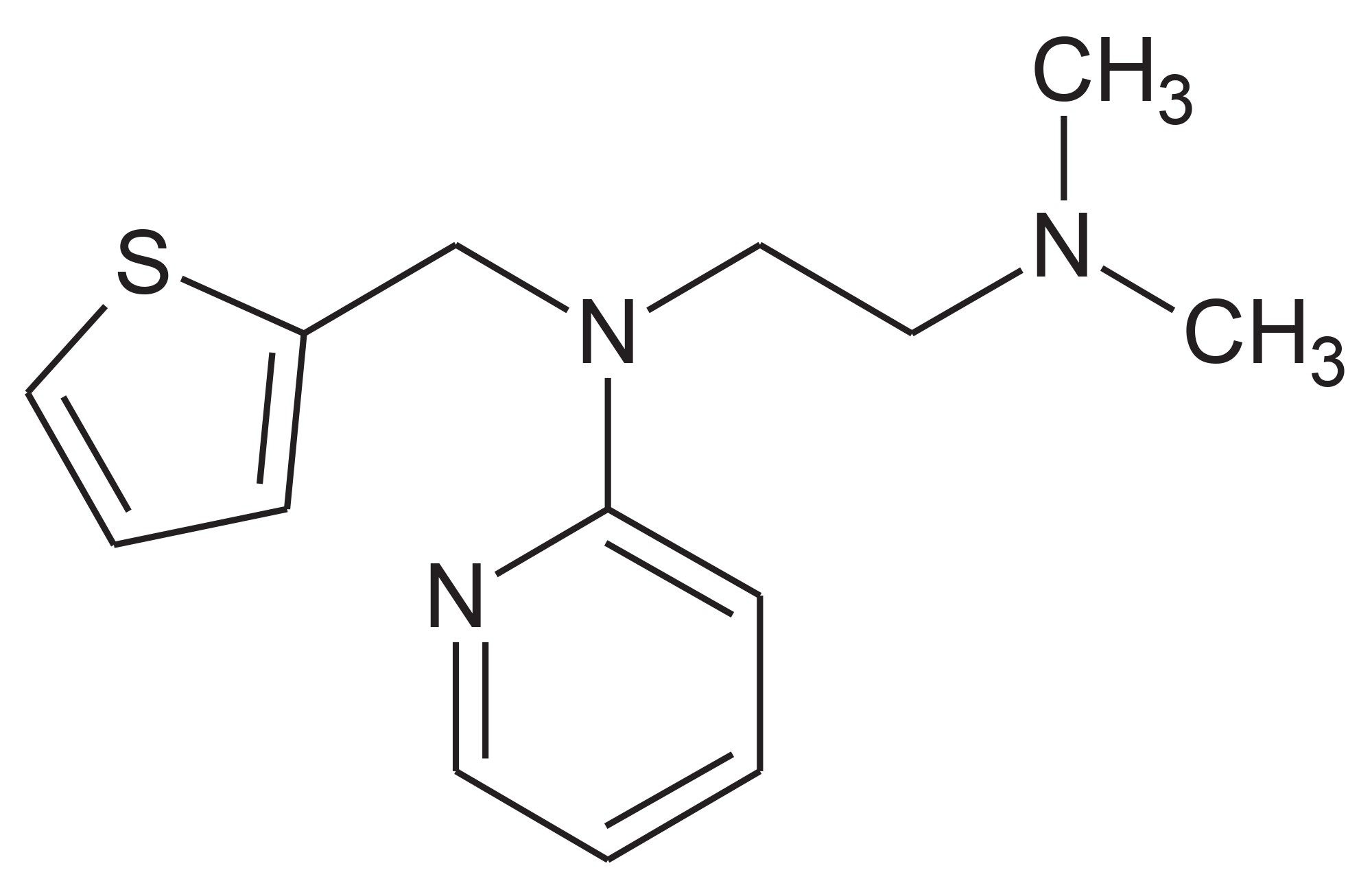
To illustrate the application of these two MS acquisition processes urine collected on day 6 (D6) following the oral administration of methapyrilene (Figure 1), an antihistamine and anticholinergic drug, to the male wistar rat at 150 mg/Kg was analysed by reversed-phase UPC-MS-MS.7 The column effluent was monitored in positive ion ESi mode using constant neutral loss or precursor ion scanning.
Constant Neutral Loss
In constant neutral loss acquisition mode, the two resolving quadrupoles of the mass spectrometer are set to scan in synchronization with a constant mass offset, this allows for the detection of all the components in the sample that give rise to the same loss irrespective of the type of sample. Sulphation and glucuronidation are the two most common form of metabolic conjugation for pharmaceutical drugs in mammalian systems. These two conjugates have constant neutral losses of 80.06 and 176.12 Da respectively and are often screened for in drug metabolism studies. This constant neutral loss mode of LC-MS screening analysis has also been used to monitor for potential toxic metabolites such as glutathione’s. These metabolites give rise to characteristic constant neutral loss of pyroglutamic acid m/z=129, GSH m/z=307 (aliphatic and benzylic thioesters) and glutamic acid m/z=147 for thioesters.
Constant neutral Loss in Methapyrilene Samples
To illustrate the use of this acquisition mode the urine sample on D6, 24-h, following the oral administration methapyrilene to the male wistar rat, was analysed by positive ion MS using the constant neutral loss of of glucuronide and sulphate conjugates. The urine sample was analysed by reversed-phase UPLC-MS/MS (see experimental section) and the column eluent was monitored by positive ion ESi using the constant neutral loss of 80 and 176 Da, Figure 2.
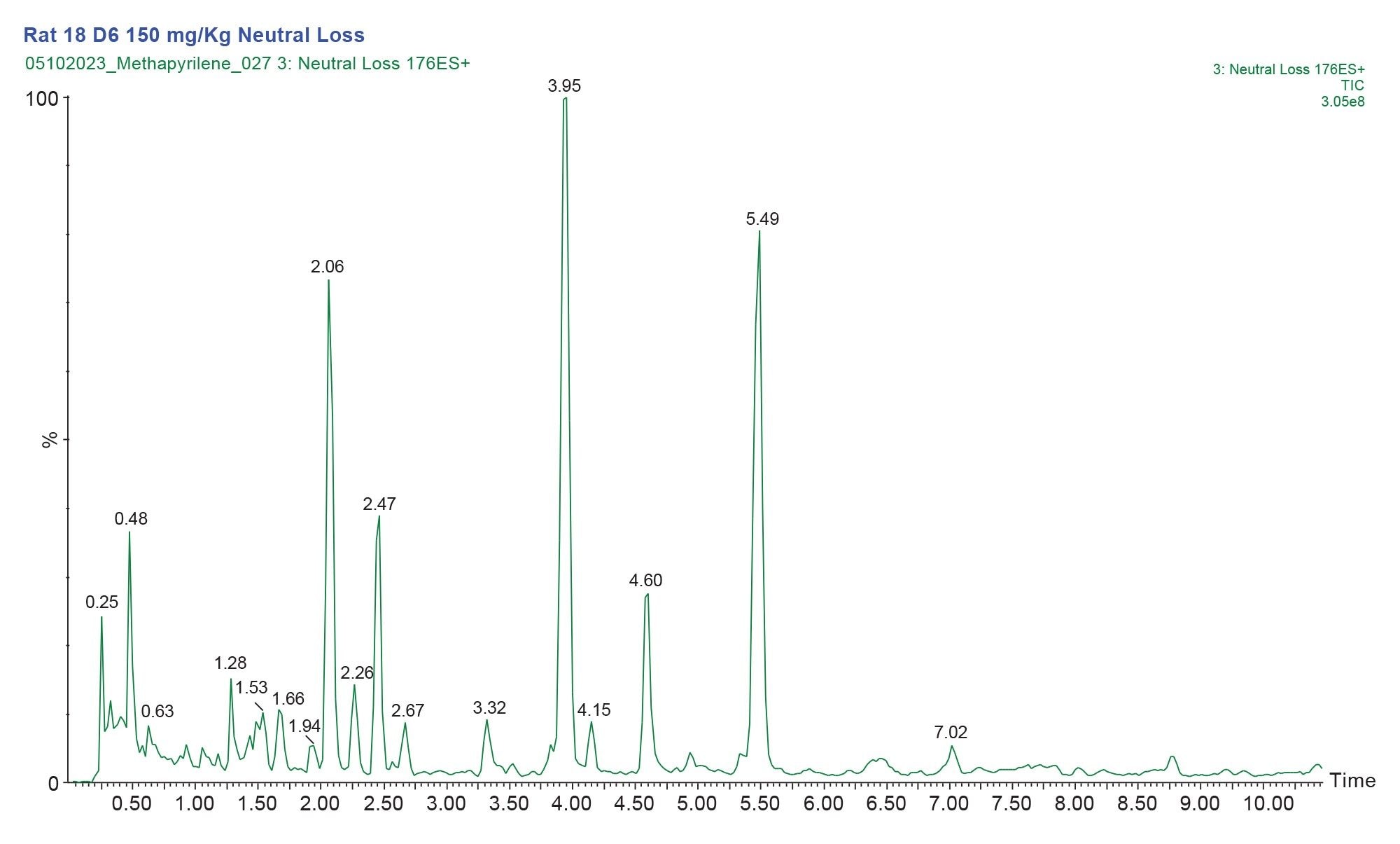
An example of the LC/MS chromatogram derived from the constant neutral loss analysis of the mass 176.12 Da is shown below in Figure 3. Here we can see that there are multiple analyte peaks which give rise to the constant neutral loss of 176 Da in the urine sample. The majority of these are not drug related and are result of the metabolism of endogenous molecules or food stuffs.
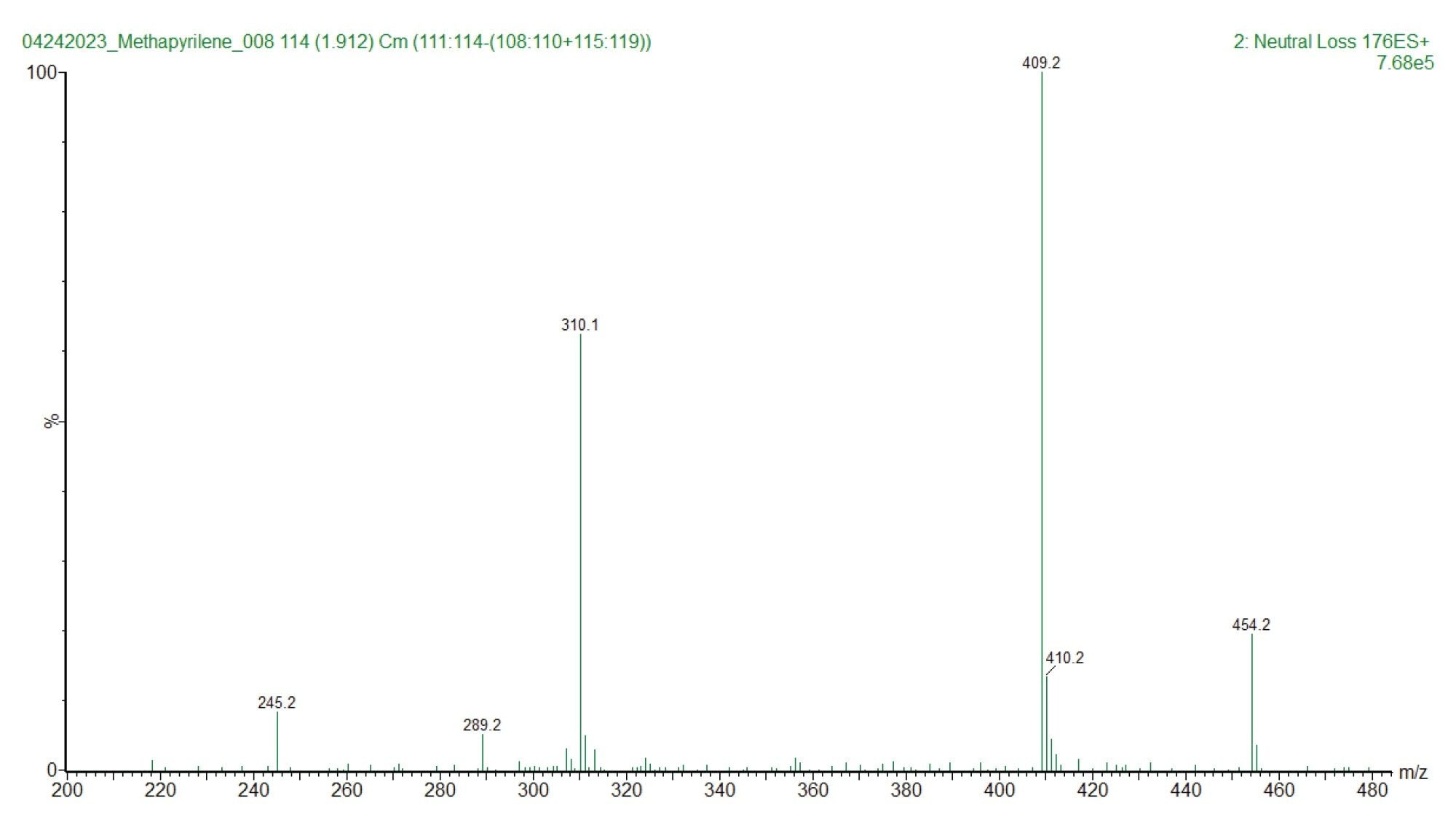
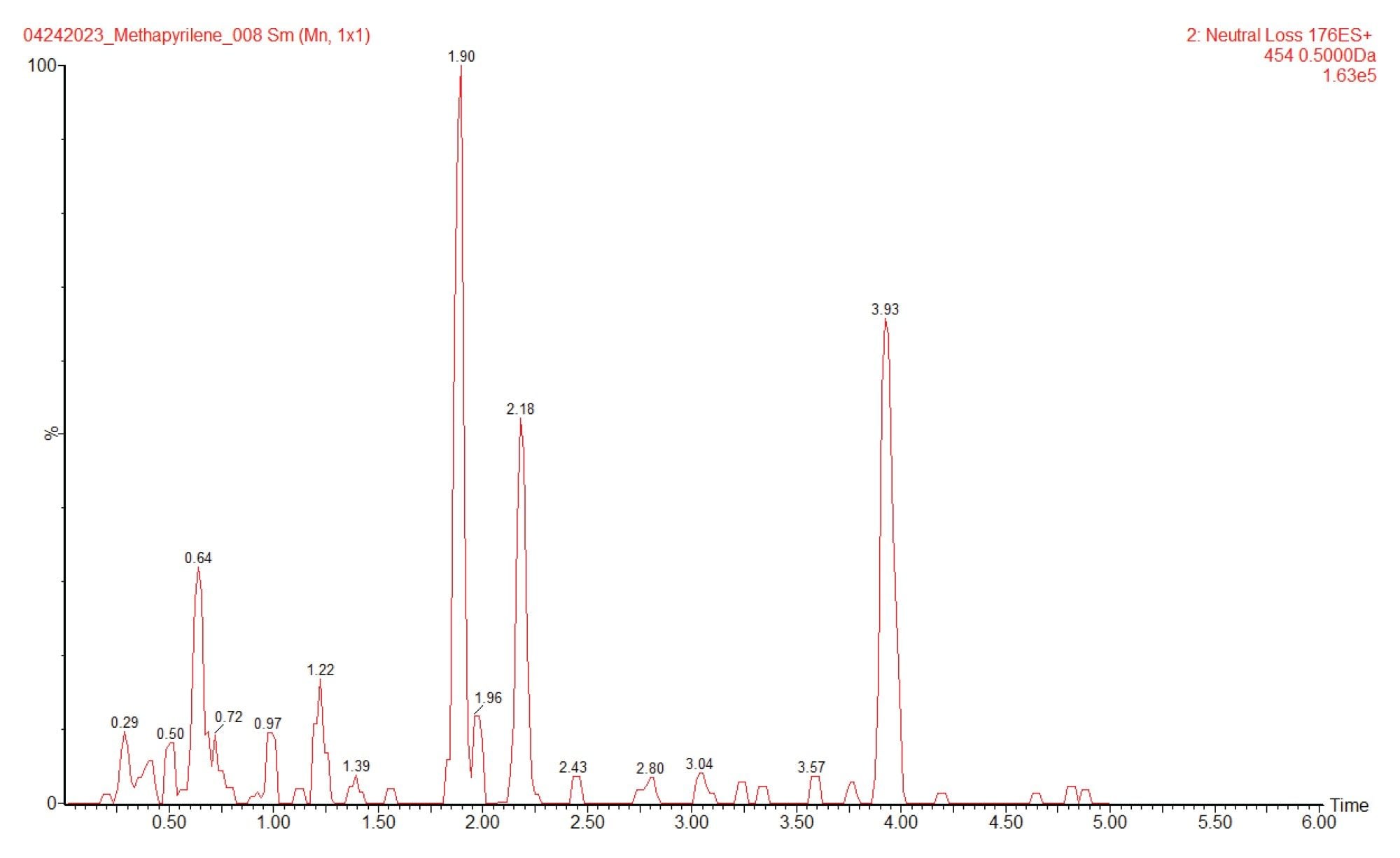
The MS1 spectrum of the peak eluting at 1.88 min is given in Figure 4. The data clearly shows the presence of a peak at m/z=454.2 suggesting that this peak is formed by the conjugation of glucuronic acid with the hydroxyl or N-oxide metabolite of methapyrilene. A targeted MS/MS analysis of this peak would be required to confirm that it is drug related. An extracted ion chromatogram, m/z=454 from the constant neutral loss data, Figure 5, indicates that there are several other peaks which are potentially drug related.
Precursor ion Scanning
Unlike constant neutral loss analysis, precursor ion scanning records all analytes which produce a specific or diagnostic fragment ion. In precursor ion scanning the first resolving quadrupole is programmed to scan over a specific mass range, all the ions within this scan range are transferred to the collision cell and subjected to fragmentation using either a fixed energy or a collision energy ramp. The fragment ions are then directed to the second resolving quadrupole which is set to transmit only ions of a specific m/z value (note, several precursor ion scans can be performed in one experiment). These specific ions are often referred to as diagnostic fragment ions as they relate to a specific fragment of the compound of interest. This mode of acquisition is often used to screen for analytes, such as drug impurities or metabolites in complex mixtures such as formulations, raw material, and biological fluids.
Precursor ion Scanning of Methapyrilene Samples
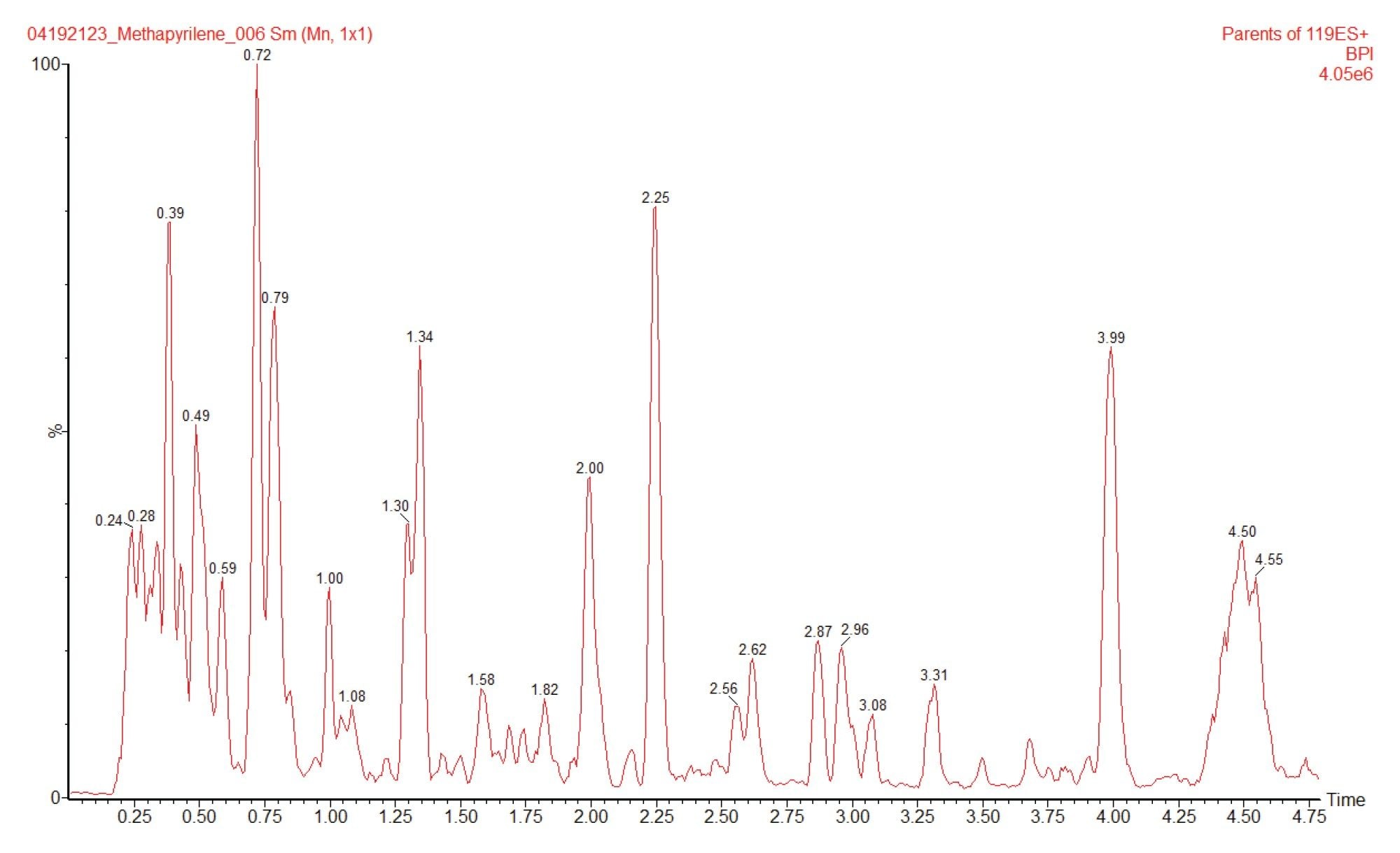
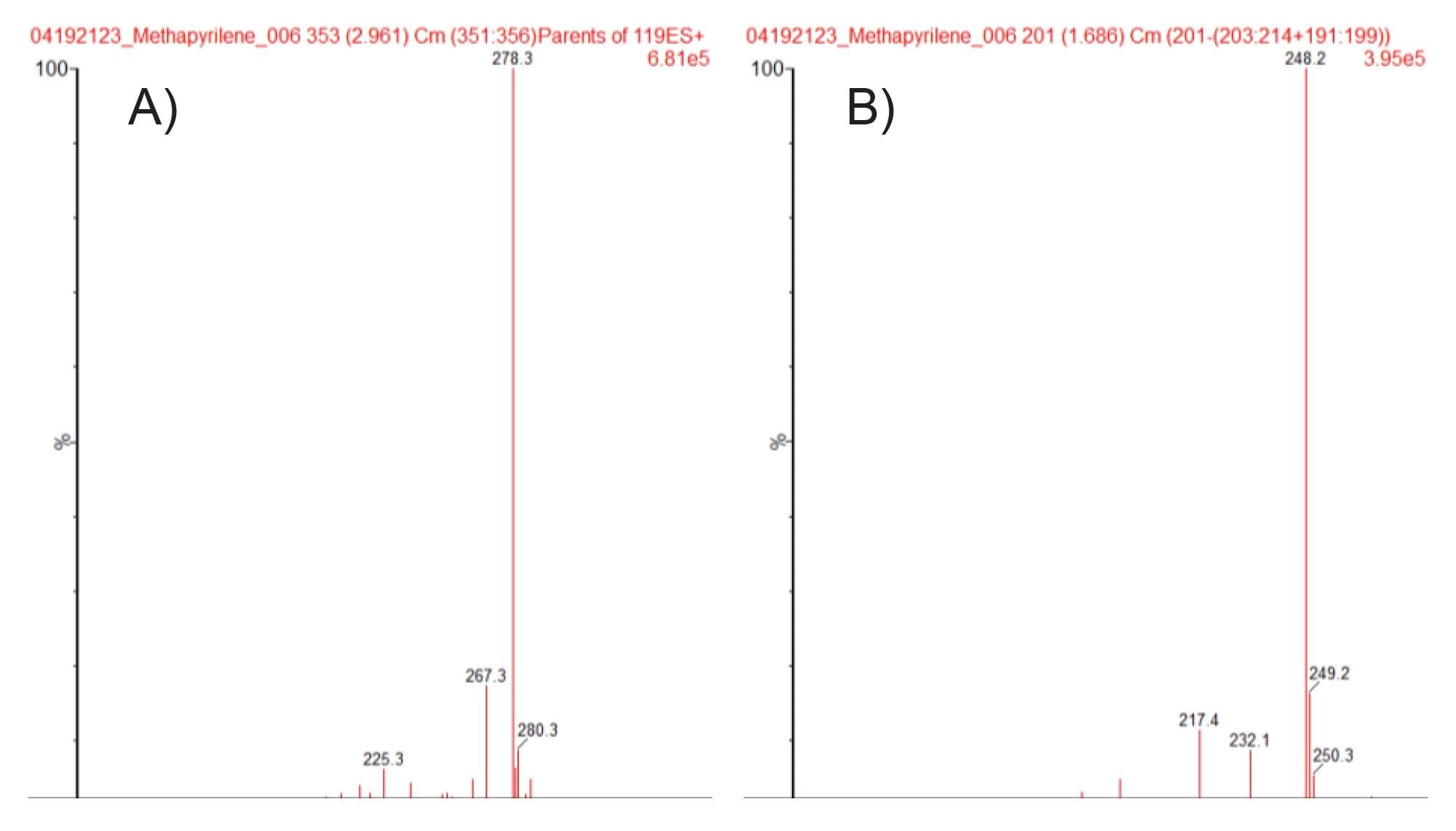
Full scan MS and MS/MS analysis of methapyrilene gave a base peak of m/z=262.2, with fragment ions m/z=217.1, 121.1, 119.1, and 96.9. Precursor ion scanning was performed using these fragment ions to interrogate the urine sample for drug related metabolites. The resulting chromatogram obtained for product ion m/z=119.1 is given in Figure 6. Multiple features were detected using this acquisition mode, integration of the chromatogram revealed the presence of several drug related peaks. As an example the MS spectra of the two peaks eluting with retention times tR=2.96 and tR=1.69 min are given in Figure 7A, B respectively. Analysis of the MS spectra of these peaks suggest that these two peaks are ; (A) the hydroxylated metabolite (m/z=278.3) and (B) the desmethyl metabolite of methapyrilene (m/z=248.2).
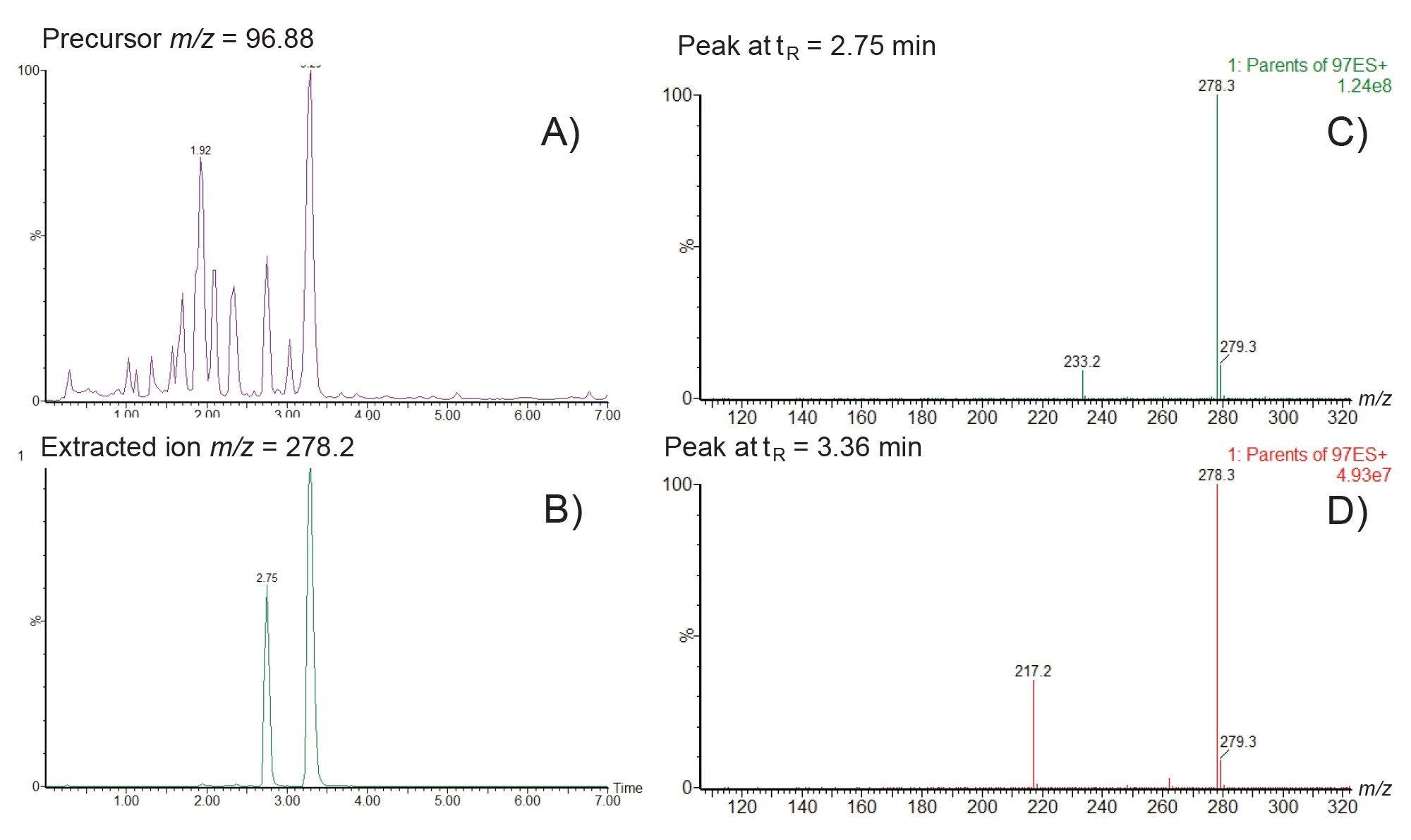
Similarly the precursor ion analysis of the fragment ion m/z=96.9 showed the presence of 11 distinct peaks eluting between 0.9–3.5 minutes, Figure 8A. The extracted ion chromatogram m/z=278.2 revealed two separate peaks eluting at tR=2.75 and tR=3.36 min, Figure 8B. Further Analysis of the MS spectra of these two peaks showed that the peak at tR=2.75 min produced an in source fragment ion at m/z 233.2. This is consistent with hydroxylation (+16Da) of the known thiopene-pyridine ring region fragment (m/z 217.2) in the parent molecule, Figure 8C. The later peak eluting peak, tR=3.36 min, did not show the presence of the m/z=217.2 fragment ion suggesting that the site of hydroxylation for this metabolite is not in the thiophene-pyridine ring region but in the tertiary amine region of the molecule. As the retention time of this peak is later than that of methapyrilene (tR=3.23 min), this peak is most likely the N-oxide metabolite of methapyrilene, Figure 8D.
Conclusion
The rapid detection and characterization of metabolic fate of a candidate drug is an essential part of drug discovery and analysis, allowing metabolic soft spots to be determined and between species toxicological coverage to be determined and potentially toxic metabolites detected. Constant neutral loss scanning and precursor ion scanning modes of data acquisition on the Waters Tandem Quadrupole Mass Spectrometer provide a rapid simple approach to evaluate in vitro or in vivo samples for the presence drug related materials. Constant neutral loss analysis identified three major glucuronide metabolites of methapyrilene in male rat urine following the administration of methapyrilene at 150 mg/Kg. Precursor ion scanning using the two fragment ions m/z=119.2 and 96.9 allowed for the facile detection of several drug related metabolites. A simple review of the data showed the presence of multiple drug related peaks including the N-oxide, desmethyl, and hydroxylated metabolites with tentative structures proposed. This data illustrates the benefits of constant neutral loss and precursor ion scanning experiments using the Waters tandem quadrupole MS for drug metabolism screening.
References
- Shu YZ, Johnson BM, Yang TJ. Role of biotransformation studies in minimizing metabolism-related liabilities in drug discovery. AAPS J. 2008;10(1):178–92. doi: 10.1208/s12248-008-9016-9.
- A rapid ultra‐performance liquid chromatography/tandem mass spectrometric methodology for the in vitro analysis of Pooled and Cocktail cytochrome P450 assays. Alden PG, Plumb RS, Jones MD, Rainville PD, Shave D. Rapid Comms Mass Spectrom. 24 (1), 147–154.
- Iwamura A, Nakajima M, Oda S, Yokoi T. Toxicological potential of acyl glucuronides and its assessment. Drug Metab Pharmacokinet. 2017 Feb;32(1):2–11. doi: 10.1016/j.dmpk.2016.11.00.
- Castro-Perez J, Plumb R, Liang L, Yang E. A high-throughput liquid chromatography/tandem mass spectrometry method for screening glutathione conjugates using exact mass neutral loss acquisition. Rapid Commun Mass Spectrom. 2005;19(6):798–804. doi: 10.1002/rcm.1855.
- Xue J, Ge L, Wang H, Liang J, Wang Q, Lu W, Cui Y, Xie H, Jian S, Jin D, Jin Q, Li T, Shen Q. Comprehensive Screening for EPA/DHA-Structured Phospholipids in Aquatic Products by a Specific Precursor Ion Scanning-Based HILIC-MS/MS Method. J Agric Food Chem. 2023 May 24;71(20):7937–7946. doi: 10.1021/acs.jafc.3c00505.
- Robert S. Plumb. Tandem Quadrupole Acquisition Modes in DMPK Studies. Waters Corporation Application Note. 720008016. September 2023.
- Graichen ME, Neptun DA, Dent JG, Popp JA, and Leonard TB (1985) Effects of methapyrilene on rat hepatic xenobiotic metabolizing enzymes and liver morphology. Fundam Appl Toxicol 5:165–174.
720008081, October 2023