Rapid Analysis of Cephalosporins and Related Drug Substances Using CORTECS™ Premier Columns featuring MaxPeak™ Technology
Abstract
Cephalosporins are medications administered to treat infections, some of which work against the worst infectious bacteria. Therefore, methods supporting the quality control of cephalosporins need to be efficient, precise, and accurate. In this application, we develop method for cephalosporins analysis, featuring CORTECS Premier Columns with MaxPeak High Performance Surfaces (HPS) Technology and compare it to a traditional stainless-steel chromatography set up. This method delivers linear, reproducible, and accurate results in under two minutes.
Benefits
- This method produces results in under two minutes for common cephalosporins
- CORTECS Premier Columns with Max Peak Technology improved chromatography, up to a 40% increase in peak height, when compared to traditional stainless-steel systems and columns
- This method is capable of quantitating actual drug samples and can be applied to the quality control sector of cephalosporins
Introduction
Cephalosporins are medications administered to treat infections.1 There are five generations of cephalosporin type drugs, some of which are the only things effective against methicillin-resistant Staphylococcus aureus (MRSA). Cephalosporins are important medications, therefore quality control testing needs to be accurate and efficient. In this application, we develop a rapid RPLC method using CORTECS Premier Columns with new MaxPeak High Performance Surfaces (HPS) Technology for the separation, and quantitation of cephalosporins. Further, we demonstrate the improvements Premier column technology provides to cephalosporins analysis when compared to a standard stainless-steel method.
It has been found that stainless steel hardware interacts with carboxylate containing analytes, such as cephalosporins, leading to unfavorable chromatography.2 As stainless-steel systems age and corrode, especially in the presence of acidic mobile phases that are commonly used for RPLC, these interactions become more apparent. Recently, Waters Corporation has released a Premier line of products featuring new MaxPeak HPS Technology. The MaxPeak HPS Technology has been shown to mitigate some of these challenges, by preventing these undesirable metal and analyte interactions.3,4,5
Here, RPLC-UV combined with MaxPeak HPS Technology refines the chromatography for cephalosporins analysis when compared to a traditional stainless-steel method. We developed a fast method that has been shown to be linear, reproducible, and accurate.
Experimental
Method of Separation Sample Description
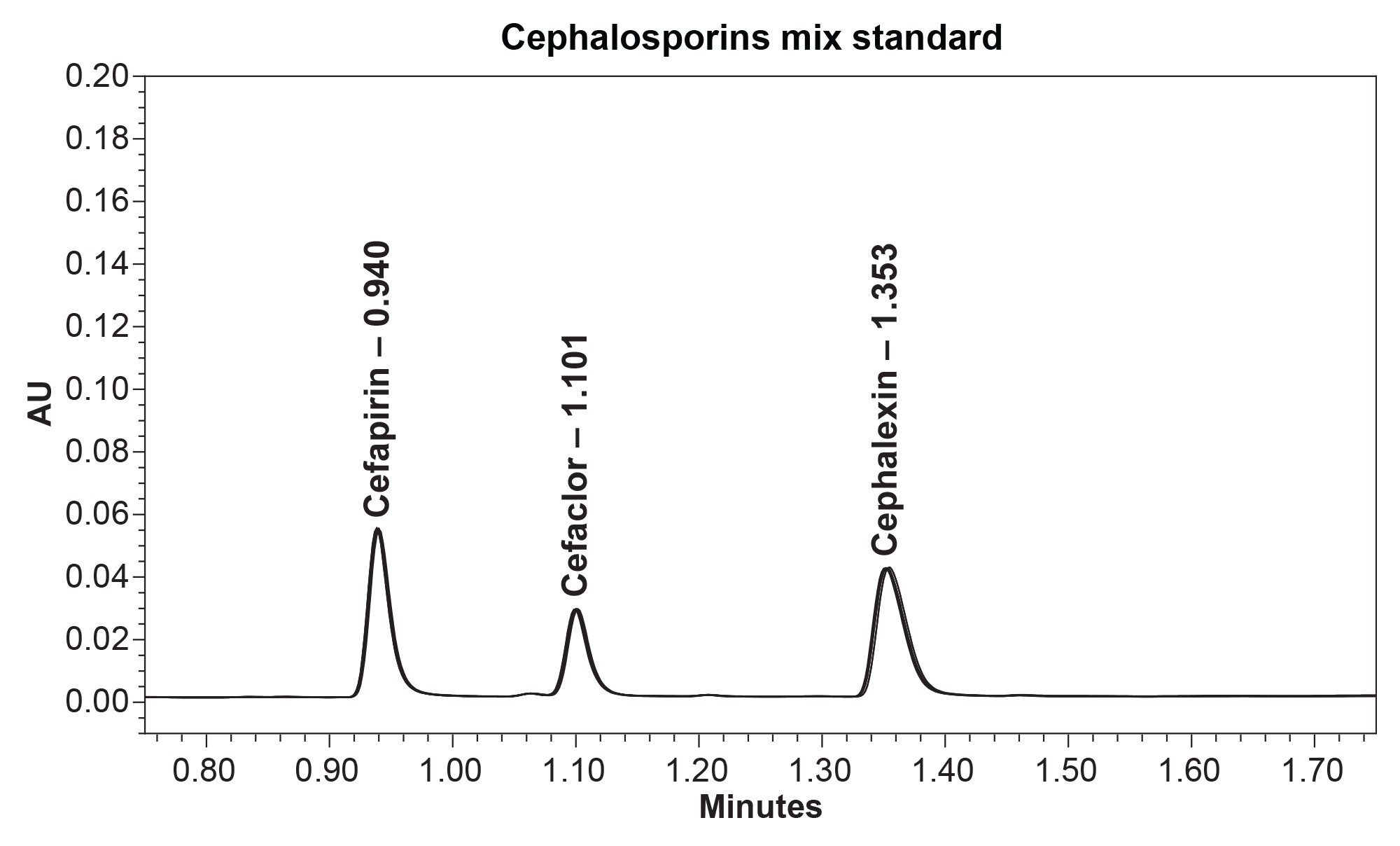
Cefapirin, cefaclor, and cephalexin were purchased from Sigma Aldrich (Milwaukee, WI). All cephalosporins were prepared as individual stocks at 1 mg/mL using 100% water as a diluent. Then stock standards were diluted and combined at a 20 µg/mL concentration in the cephalosporins mix standard. Stock solutions were stored at 2 °C–8 °C and allowed to equilibrate to ambient room temperature prior to analysis.
Linearity Sample Description
The cephalexin curve stock standard was prepared at a 1 mg/mL concentration and diluted using 100% water into a 10 ml volumetric flask. Then, various calibration standards were prepared from the stock ranging from 1 µg/mL to 100 µg/mL. Stock solutions were stored at 2 °C–8 °C and allowed to equilibrate to ambient room temperature prior to analysis.
Method Conditions
LC Conditions
Two instrument set-ups were used in this study. An ACQUITY™ Premier set up to showcase the MaxPeak HPS Technology, and a traditional ACQUITY UPLC stainless-steel set up was used to compare against the Premier setup. Each system was ran using the same instrument conditions, just different components.
Instrument Set-up
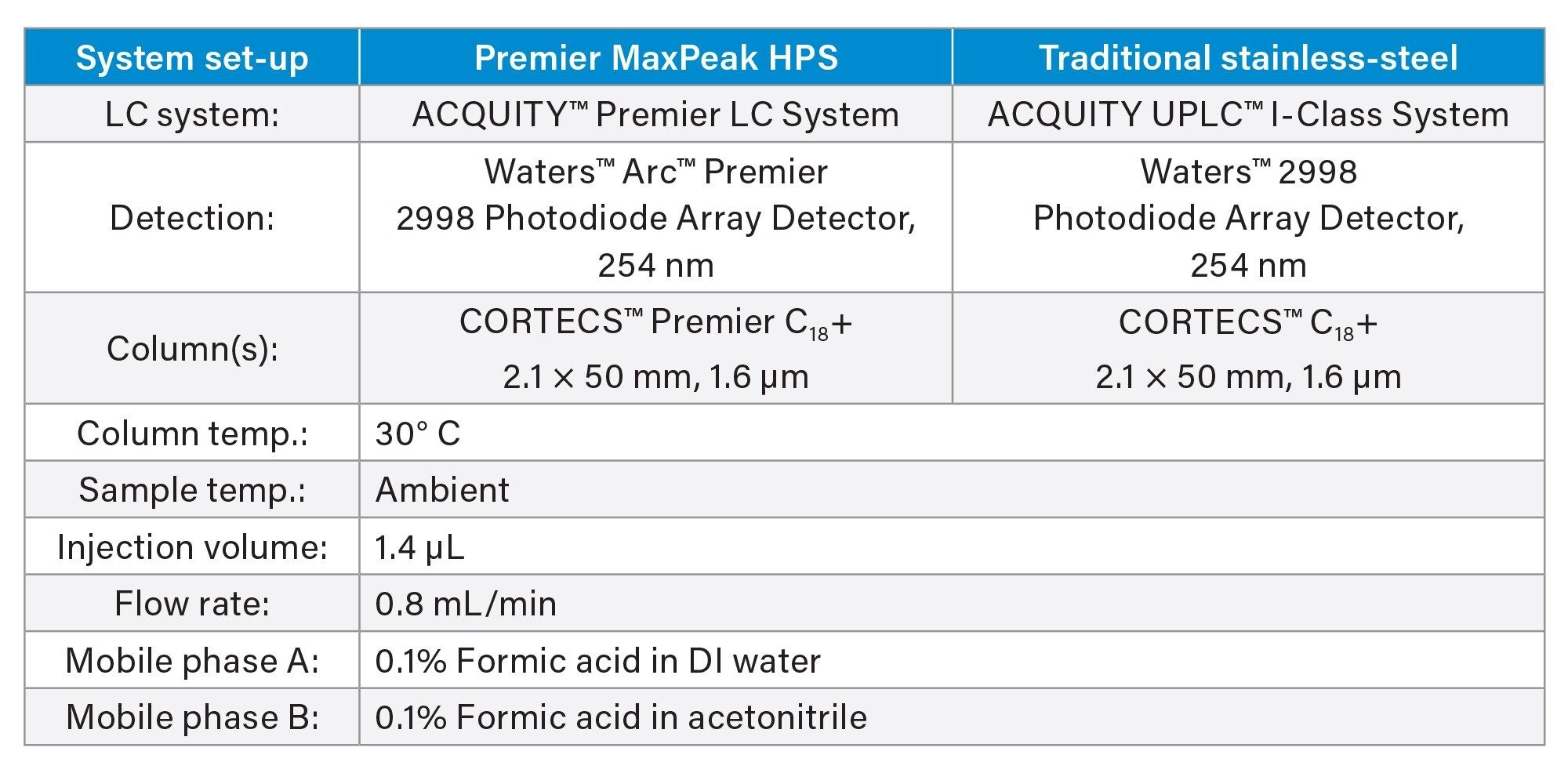
Gradient Table
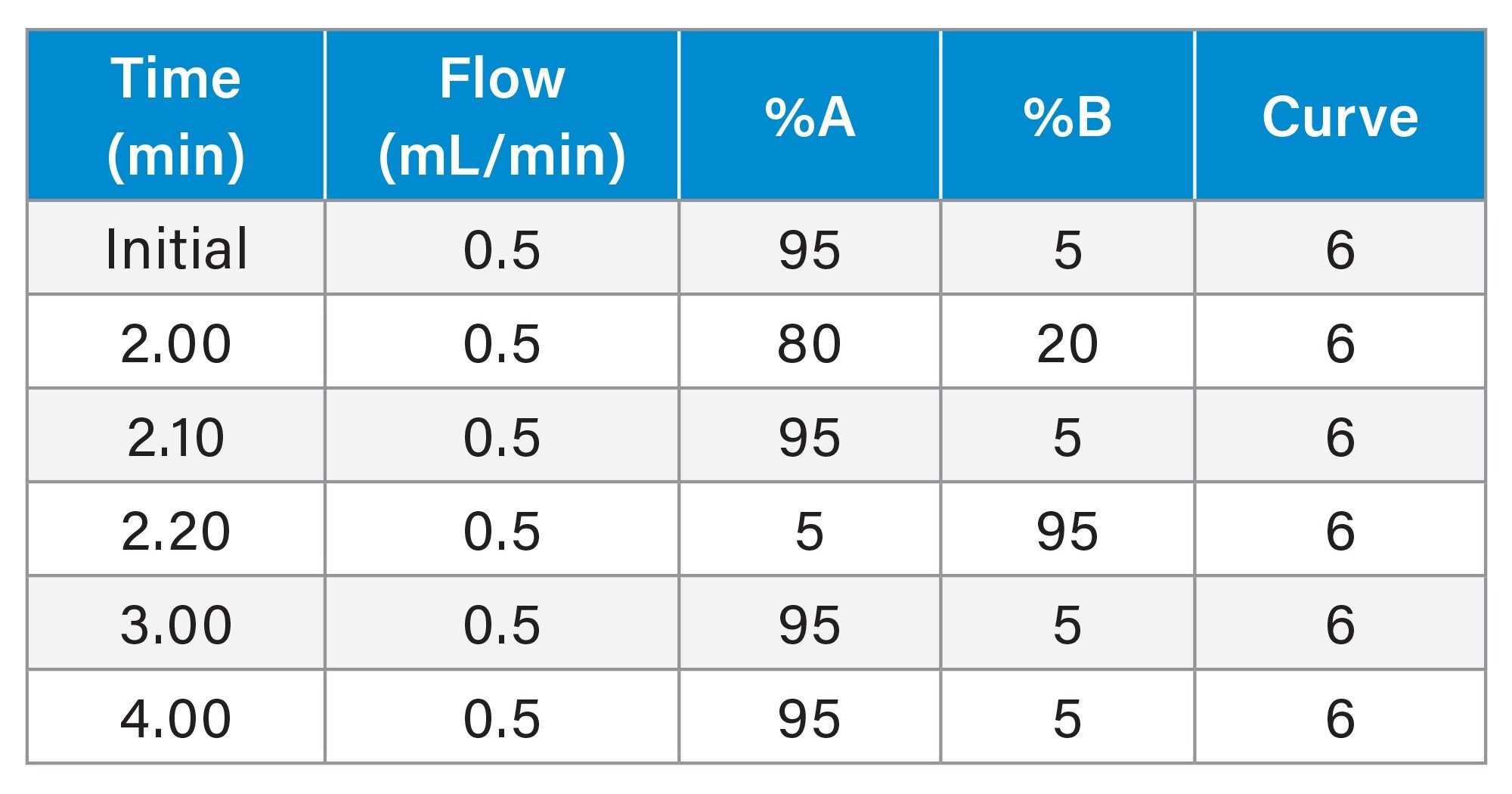
Data Management
|
Chromatography software |
Empower™ 3 Software Build 3471 |
Results and Discussion
Method of Separation Results
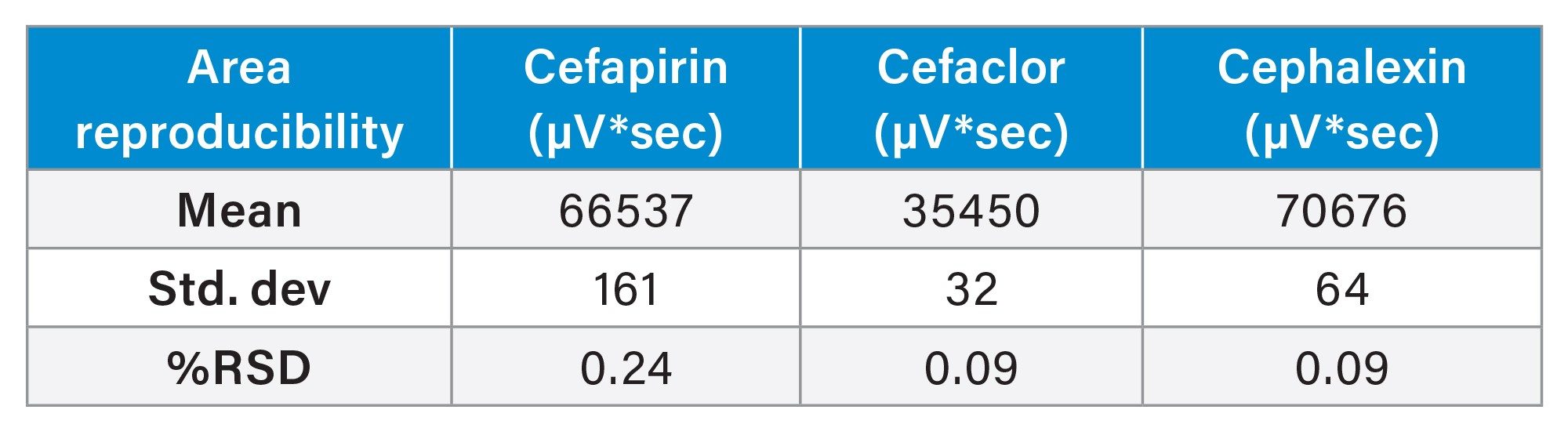
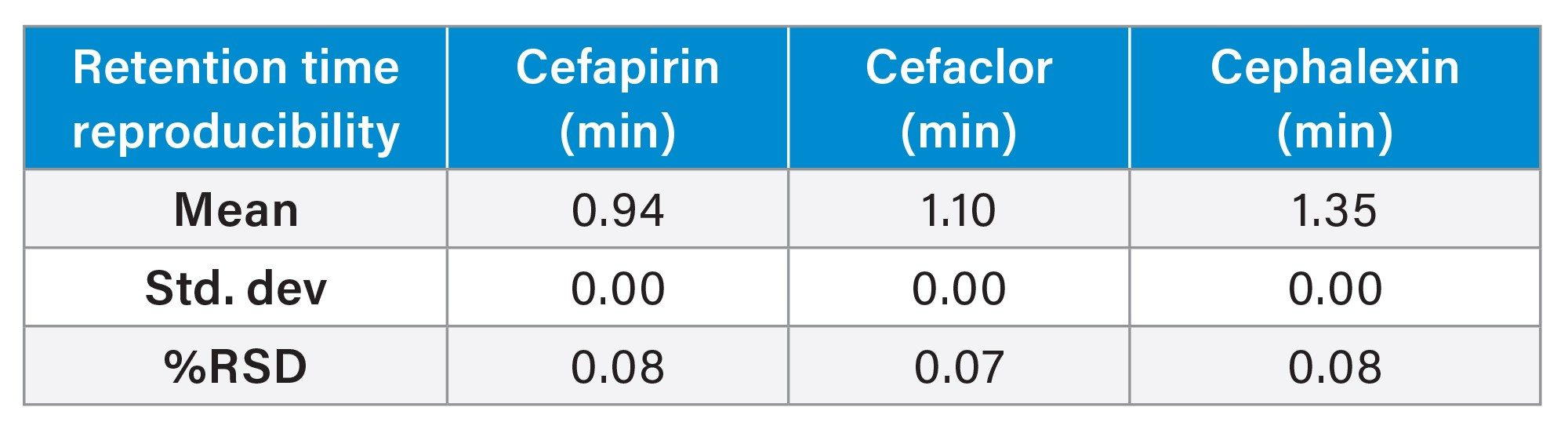
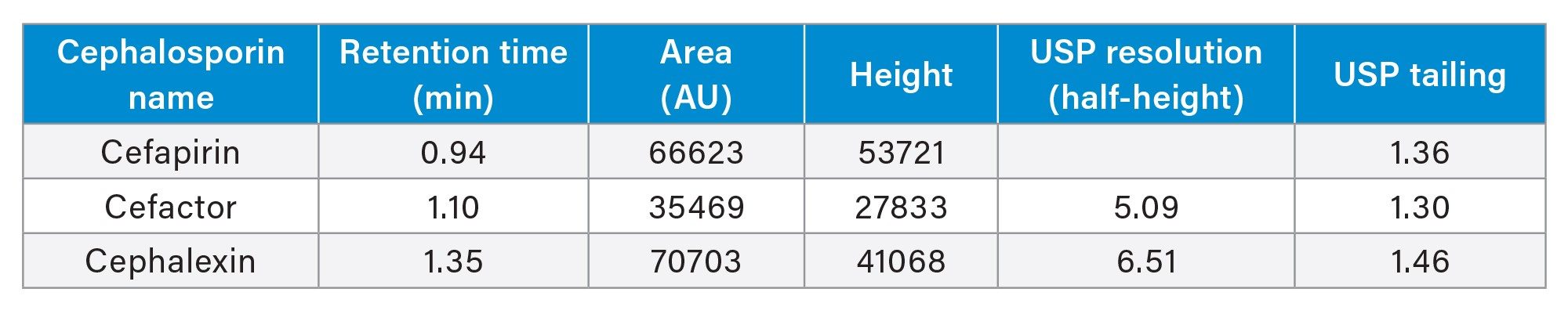
This method reproducibly separates and retains three common cephalosporins. After ten injections, the %RSD for area and retention time for cephalosporins were <5% (Table 1 and Table 2). Below, an overlay chromatogram of the ten injections provide a clear picture of the method’s performance (Figure 1a).
Linearity Results
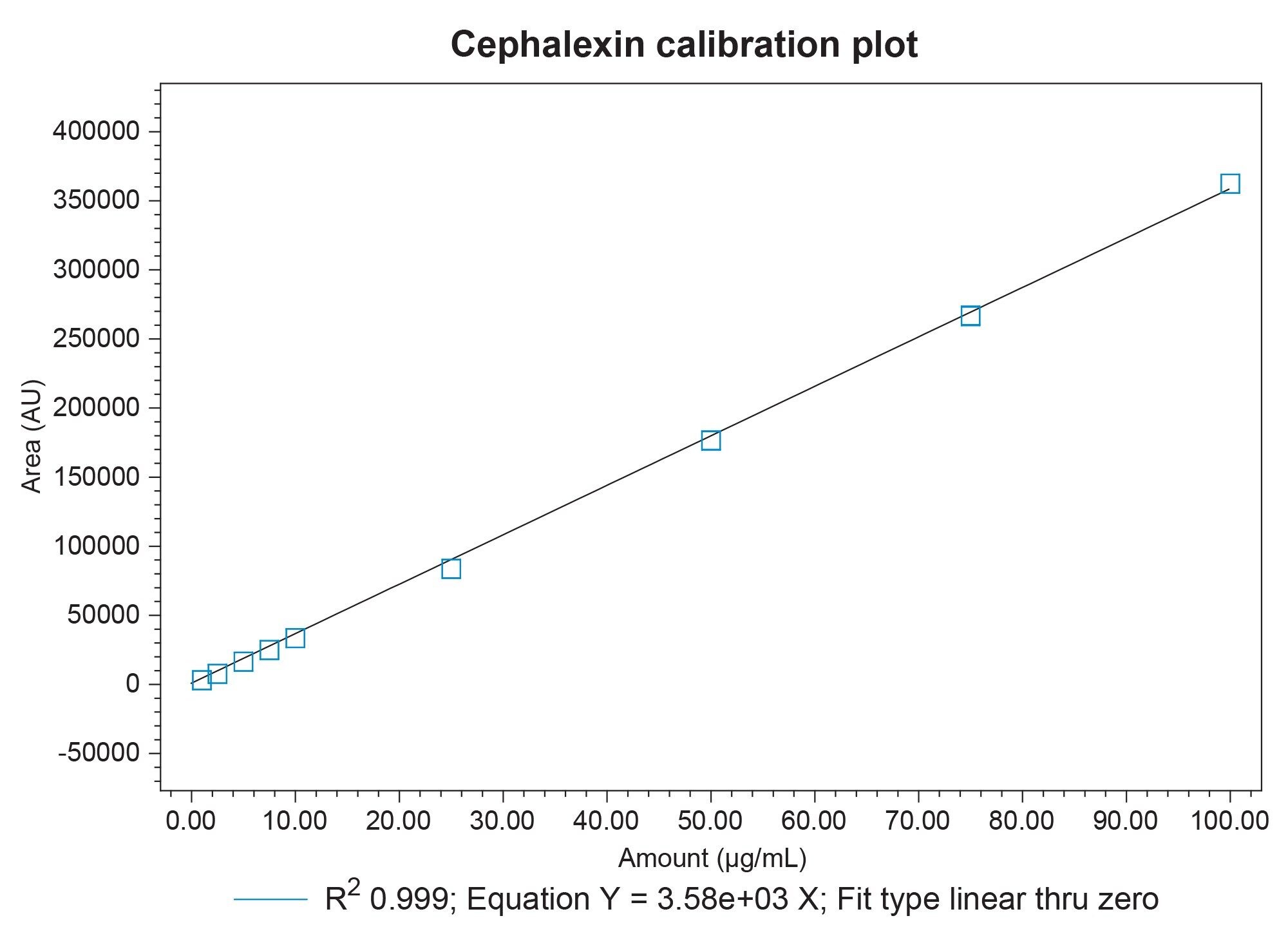
Linearity was performed on cephalexin to demonstrate the quantitative suitability for this method. The linear data collected supports its use for quality control testing (Figure 2).
System Comparison Results
This method was transferred on to a stainless-steel system to highlight the improvements that CORTECS Premier Columns with MaxPeak HPS Technology has on cephalosporins analysis. Ten injections of the cephalosporins mix standard were performed using the same standard on each of the instrument set ups. In figures 3a and 3b below, both systems can run the method successfully.
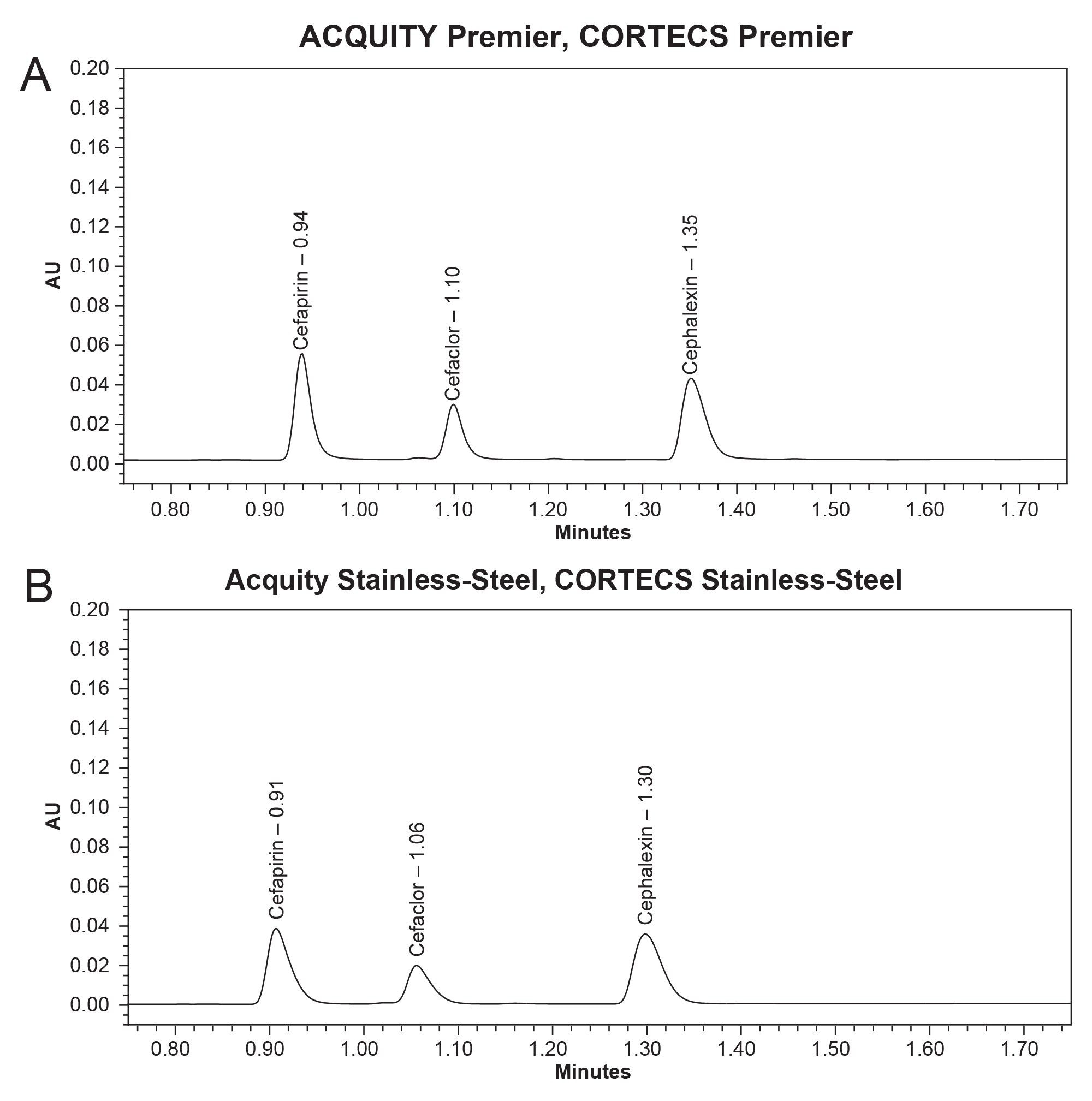
Figure 3b. Chromatogram for injection five out of ten of the cephalosporins mix standard on the ACQUITY I-Class System equipped with a CORTECS C18+ Column.
The chromatographic data for figures 3a and 3b is detailed in tables 3 and 4, respectively. Here, a clear picture of the benefits of CORTECS Premier Columns with HPS MaxPeak Technology is shown. Between the two systems premier delivers up to a 40% increase in height signal. Clearly this technology offers clear improvements in the retentivity, separation, peak symmetry when compared to a traditional stainless steel set up for cephalosporins analysis.

Figure 3b. Chromatogram for injection five out of ten of the cephalosporins mix standard on the ACQUITY I-Class System equipped with a CORTECS C18+ Column.
Sample Quantitation Results
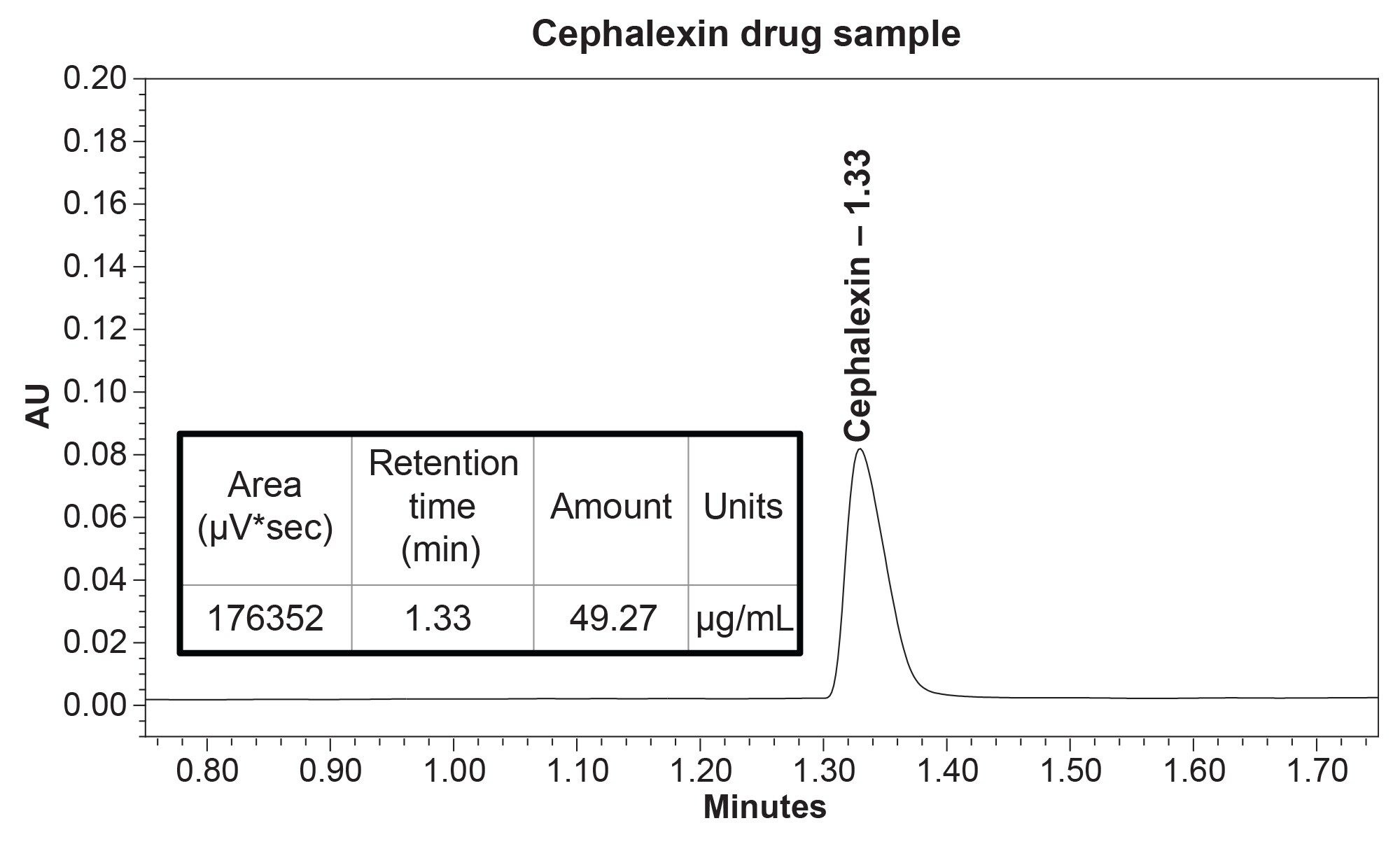
To demonstrate the quantitative application of this method, a cephalexin drug sample was prepared at a 50 µg/mL concentration. When analyzed, the sample was quantitated at 49.27 µg/mL, giving a 98% recovery (Figure 4).
Conclusion
Here, the use of CORTECS Premier Columns with MaxPeak HPS Technology improved the analysis of cephalosporins when compared to traditional stainless-steel chromatography instruments and columns. The method produced is efficient and delivers reproducible analytical results in less than two minutes per injection. Further, the method is linear and has potential to be used for accurate quantitative analysis of cephalosporins. In conclusion, CORTECS Premier Columns with MaxPeak HPS Technology enhances cephalosporins analysis when compared to a traditional stainless steel chromatography setup.
References
- Bui T, Preuss CV. Cephalosporins [Internet]. PubMed. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2022 Sep 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551517/.
- Walter T, Trudeau M, Simeone J, Rainville P, Patel A, Lauber M, Kellett J, DeLano M, Brennan K, Boissel C, Birdsall R, Berthelette K. Low Adsorption UPLC Systems and Columns Based on MaxPeak High Performance Surfaces: The ACQUITY Premier Solution. Waters Corporation; Available from: 720007128, 2021 May.
- Layton C, Rainville P. Improvements in Chromatographic Performance for Stability Indicating Methods of Antiviral Drugs With MaxPeak Premier Technology. Waters Application Note; 720007487, 2022 Jan.
- Shah D, Smith K, Yang J, Hancock P. Analysis of Fourteen Organic Acids in Various Beverages Using the ACQUITY UPLC H-Class PLUS and ACQUITY QDa Mass Detector. Waters Application Note; 720007289, 2021 Aug.
- Waters Corporation. CORTECS Columns, Applications Notebook. Available from: 720004739, 2012.
720007750, January 2023