Absolute Analytical Sensitivity Utilizing the new Xevo™ TQ Absolute IVD for the UPLC™-MS/MS Analysis of a Panel of Steroid Hormones for Clinical Research
For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
This application brief demonstrates the high analytical sensitivity and quantitative performance of the Xevo TQ Absolute IVD mass spectrometer for the analysis of a panel of steroid hormones for clinical research using only 100 μL of serum.
Benefits
- Utilizing the Xevo TQ Absolute IVD for low level quantification of steroid hormones
- Simple SPE sample preparation method
- UPLC separation of isobaric compounds for selective detection
Introduction
Steroid hormones play a central role in many biochemical processes, which include controlling metabolism, inflammation, immune functions, salt and water balance, development of sexual characteristics, and the ability to withstand illness and injury. Enzymes that form part of the steroid biosynthesis pathway are pivotal in these metabolic processes and their dysfunction can be examined for clinical research through steroid hormones in the pathway.
Measurement of these steroids by immunoassay is prone to analytical interference as a result of cross reactivity of assay antibodies with structurally related steroid hormones and synthetic derivatives. Liquid chromatography – tandem mass spectrometry (LC-MS/MS) can provide analytically sensitive, accurate and precise measurement of these steroid hormones.

Enhanced analytical sensitivity, robustness, and reliability over six orders of linear dynamic range is achieved using the Xevo TQ Absolute IVD mass spectrometer, featuring StepWave™ XS ion transfer optics and an Xtended Dynamic Range (XDR). Coupling this with a simple sample preparation method with the MassTrak™ Endocrine Steroid Calibrator and Quality Control Sets, utilizing Oasis™ MAX µElution™ technology, and UPLC separation of the steroid hormones allows for a highly selective and analytical sensitivity method to analyze steroid hormones for clinical research. The steroid hormones investigated were androstenedione, 17-hydroxyprogesterone (17-OHP), dehydroepiandrosterone (DHEA), progesterone, dihydrotestosterone (DHT), and testosterone.
Experimental
Sample Preparation and UPLC-MS/MS Analysis
Sample extraction was performed using a Hamilton STAR liquid handling robot. To 100 μL of MassTrak Endocrine Steroid Calibrators, QCs and samples, internal standard was added, and samples were precipitated using methanol and water, mixing after each reagent addition. During centrifugation of samples, an Oasis MAX μElution Plate (p/n: 186001829) was conditioned and equilibrated. An aliquot of each of the pre-treated samples was loaded into individual wells and slowly pulled through the plate. Consecutive washes with formic acid in 15% acetonitrile(aq) and ammonia in 15% acetonitrile(aq) were performed to reduce potential ionic interference. Analytes were eluted using 60% acetonitrile(aq), followed by the addition of water.
Samples were subsequently injected onto an ACQUITY UPLC I-Class FL System and Xevo TQ Absolute IVD Mass Spectrometer, utilizing a water/methanol/ammonium fluoride gradient and a Waters™ CORTECS™ UPLC C18 Column.
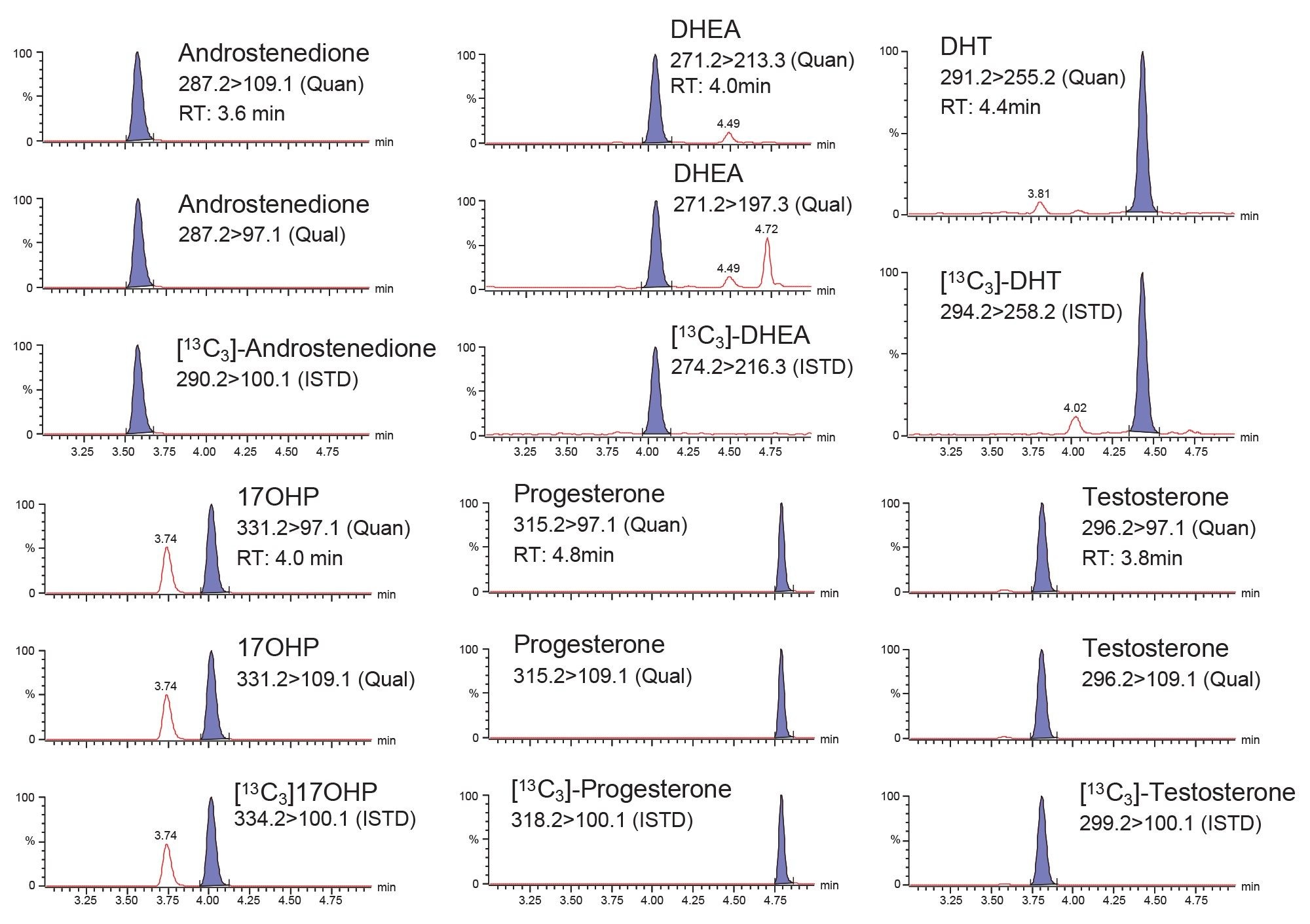
Multiple Reaction Monitoring (MRM) transitions, quantifier (quan), and qualifier (qual), for the detection of all analytes are shown in Figure 2.
Results and Discussion
Chromatographic separation was achieved for all isobaric steroid hormones, having an injection- to- injection time of approximately 7.0 minutes. A typical chromatogram is shown in Figure 2.
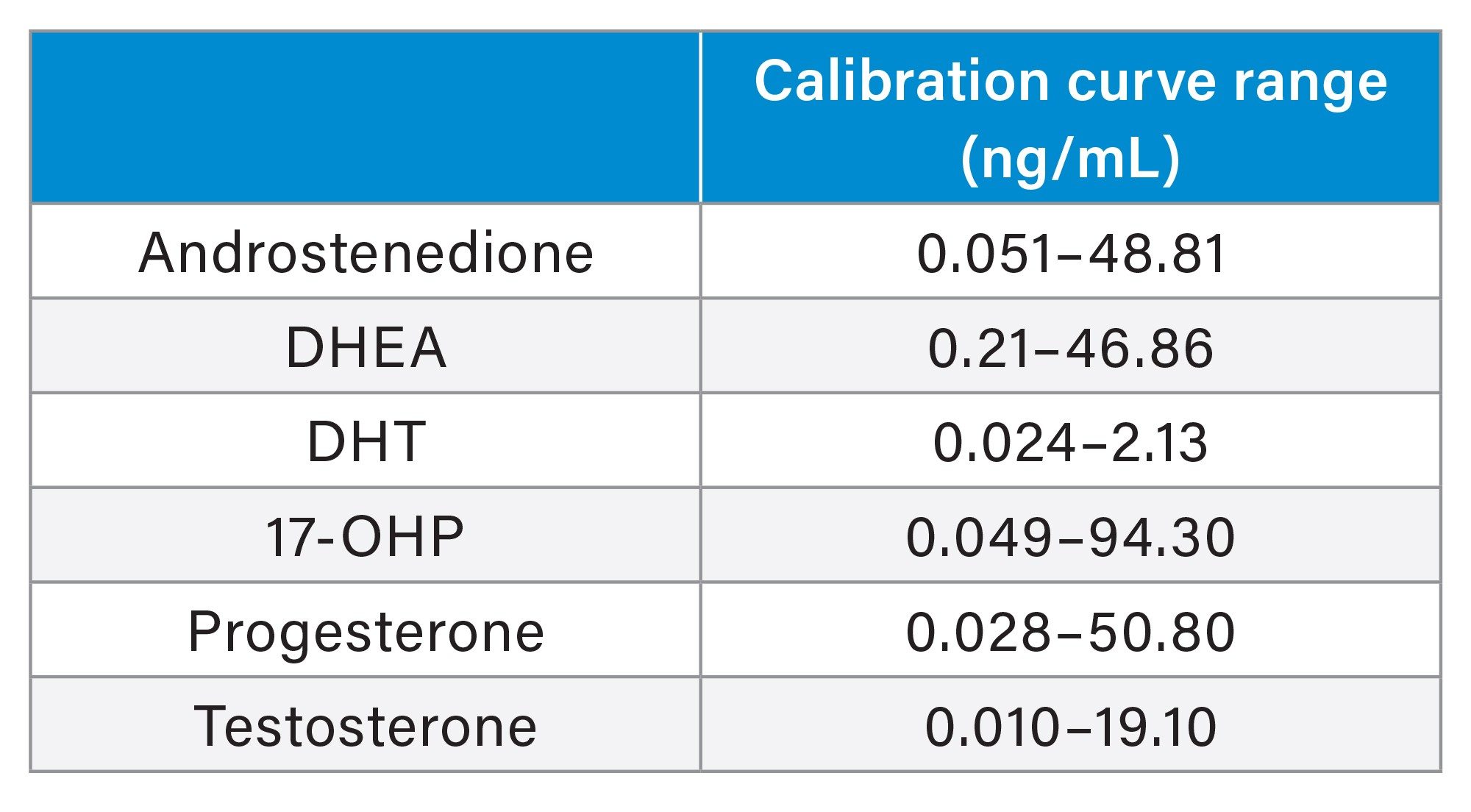
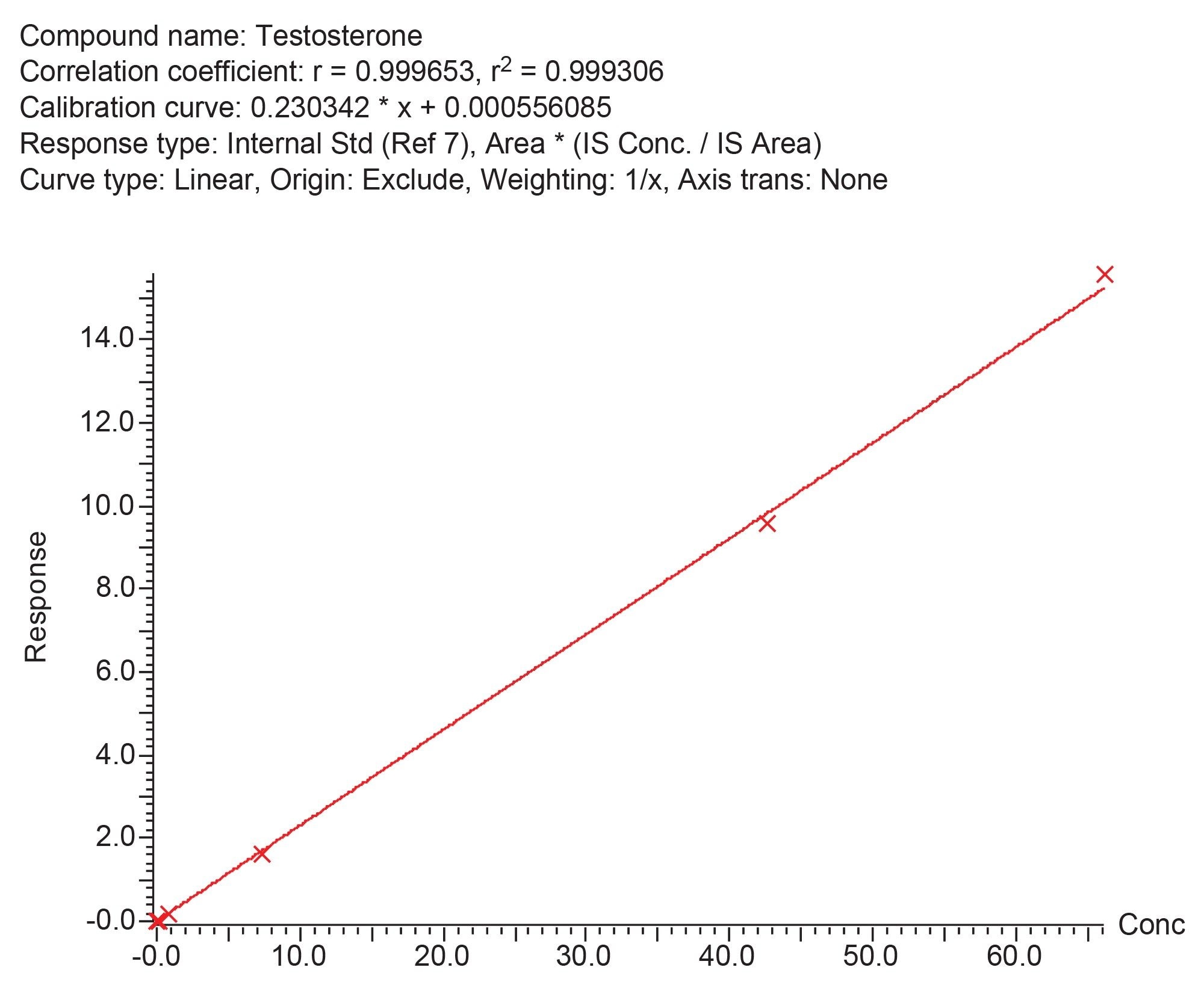
Calibration curves were shown to be linear over the calibration ranges shown in Table 1, having correlation coefficients of >0.99 and %bias of within ±15% (±20% for Calibrator 1) for all analytes across five occasions. An example calibration curve is shown in Figure 3.
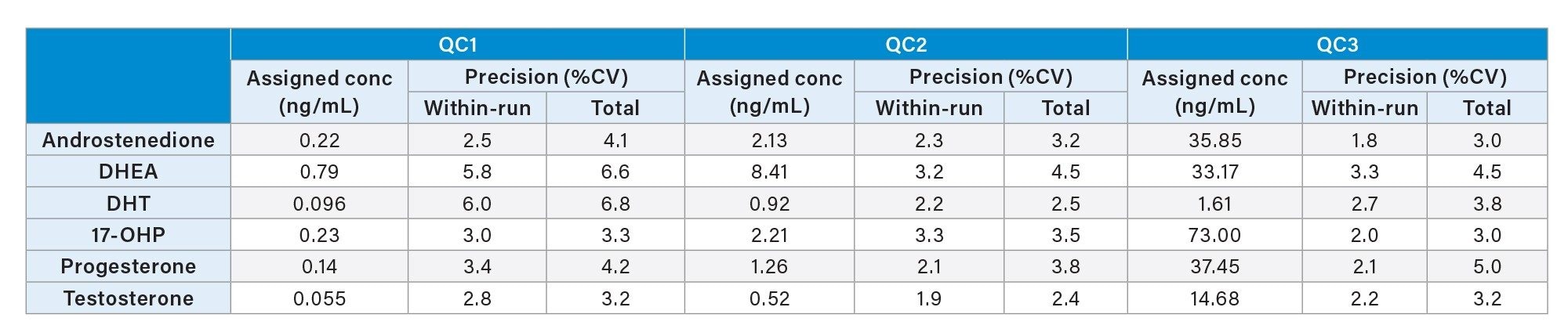
Precision performance was assessed by extracting and analyzing five replicates of the low, mid and high MassTrak Endocrine QC Set on each of five occasions. Within-run and total precision were ≤6.8% CV for all steroid hormones for all concentration levels and is summarized in Table 2.
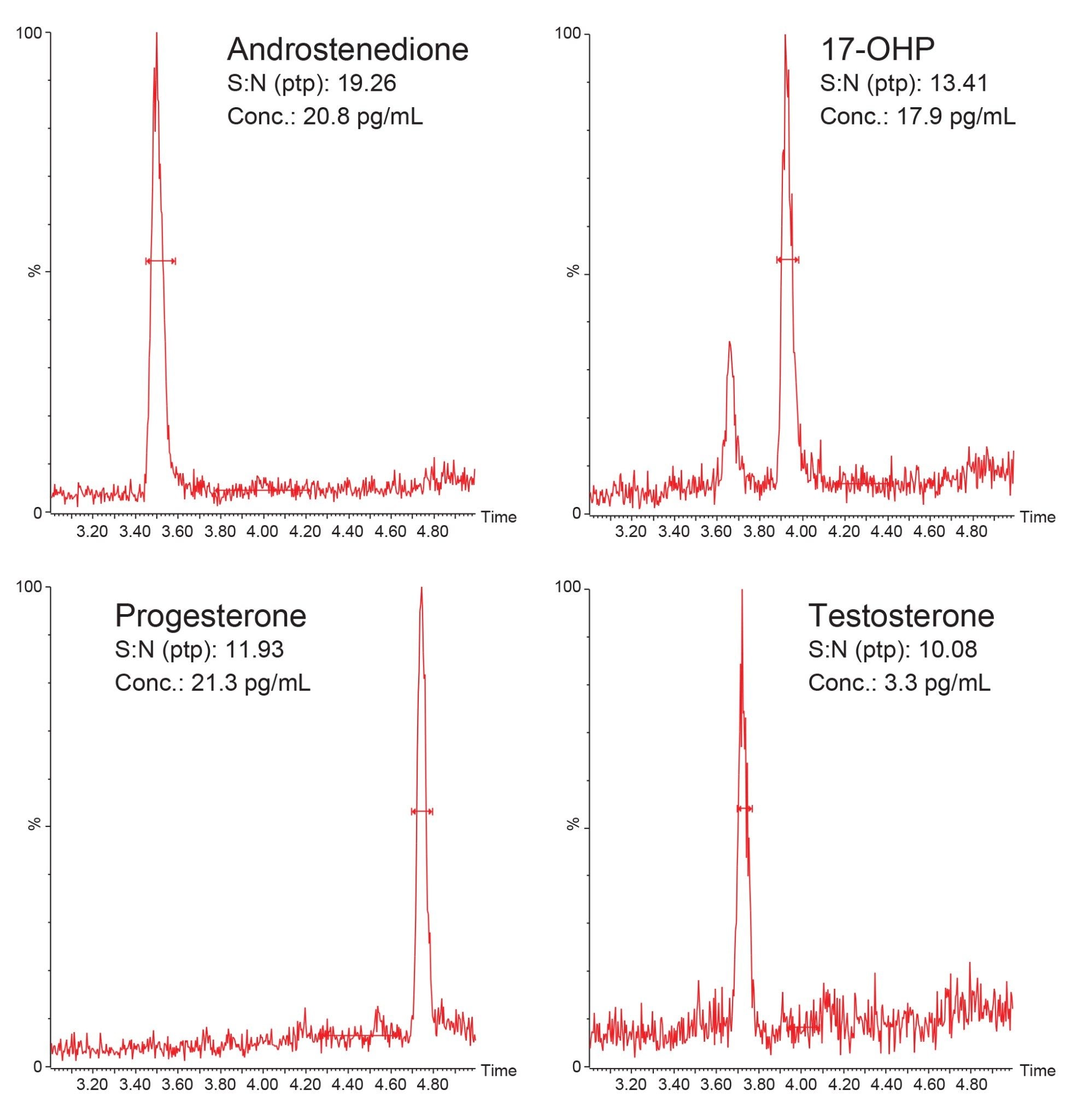
Analytical Sensitivity was assessed by diluting the Calibrator 2 solution with Golden West Biologicals MSG4000 stripped serum to create low level pool samples. Ten replicates of each sample were then extracted and analyzed on each of four occasions and the %CVs were calculated and shown in Table 3. %CVs of ≤20% were obtained at 20.8 pg/mL for Androstenedione, 17.9 pg/mL for 17-OHP, 11.9 pg/mL for Progesterone, and 3.3 pg/mL for Testosterone. Figure 4 also shows typical chromatograms of each analyte at these concentrations.

Conclusion
The Xevo TQ Absolute IVD has demonstrated excellent analytical sensitivity and quantitative performance for the analysis of a steroid hormone panel for clinical research, having the following method performance characteristics:
- Calibration curves had correlation coefficients (r2) of >0.99 for all steroid hormones for all runs.
- Within-run and total precision results of ≤6.8% CV.
- Analytical sensitivity concentrations of 20.8 pg/mL for Androstenedione, 17.9 pg/mL for 17-OHP, 11.9 pg/mL for Progesterone, and 3.3 pg/mL for Testosterone were achieved, having a %CV of <20% and S:N (ptp) of >10:1 from only 100 µL of serum.
For Research Use Only. Not for use in diagnostic procedures.
720007892, March 2023