LC-MS/MS Analysis of Amyloid Beta Peptides in Artificial Cerebrospinal Fluid Using the Xevo™ TQ Absolute Mass Spectrometer for Clinical Research
This is an Application Brief and does not contain a detailed Experimental section.
For research use only. Not for use in diagnostic procedures.
Abstract
Amyloid-beta (Aβ) peptides with sequences containing 36–43 amino acids are the main component of amyloid plaques deposited in the brains of individuals with neurodegenerative disorders, therefore, these peptides are of interest in clinical research. In this application brief, we demonstrate the suitability of the ACQUITY™ Premier UPLC I-Class System with Xevo™ TQ Absolute Mass Spectrometer as a tool in clinical research for analytically sensitive and selective quantitation of multiple Aβ peptide isoforms (1–38, 1–40, 1–42) in artificial Cerebrospinal Fluid (aCSF).

Introduction
Amyloid-beta (Aβ) peptides with sequences containing 36–43 amino acids are the main component of amyloid plaques deposited in the brains of individuals with neurodegenerative disorders, therefore, these peptides are of interest in clinical research of pharmacodynamics investigations of new therapeutics. Historically, quantification of Aβ peptides in biological fluids has relied mainly on the use of immunoassays, such as ELISA.1,2 These techniques can suffer from cross-reactivity, contributing to batch-to-batch variation of the methods, which can impact confidence in results. In addition, for assessments that involve multiple biomarkers, an individual ELISA method is required for each peptide, increasing overall analysis time and cost. Therefore, a single robust method providing greater analytical selectivity would help overcome these challenges. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) could be a beneficial technique in the clinical research of Aβ peptides. These benefits include improvements in analytical selectivity, and the capability of multi-analyte quantitative detection in a single run. Utilizing the surrogate matrix methodology, the Aβ peptide isoforms can be measured with selectivity achieved through sample preparation, chromatographic separation, and Multiple Reaction Monitoring (MRM) mass detection.
Herein, we demonstrate the suitability of the ACQUITY™ Premier UPLC I-Class System with Xevo™ TQ Absolute Mass Spectrometer as a tool in clinical research for analytically sensitive and selective quantitation of multiple intact Aβ peptide isoforms (1–38, 1–40, 1–42) extracted from 200 µL of artificial Cerebrospinal Fluid (aCSF) over the concentration range 0.1–10 ng/mL.
Sample Preparation and LC-MS Analysis
The sample preparation and LC-MS analysis details from the Application Note 720006517 were used with some minor changes highlighted in this section to improve analyte retention.3
Calibration and Quality Control (QC) working solutions at each level were spiked into the blank artificial CSF with 0.4% (w/v) Bovine Serum Albumin (BSA). An internal standard working solution was added to 200 µL of spiked samples, which was diluted with 200 µL of 5 M guanidine-HCl and 200 µL of 4% (v/v) phosphoric acid. After incubation at room temperature for one hour the samples were loaded onto an Oasis™ MCX SPE µElution plate. The plate was washed and subsequently eluted using the protocol in 720006517 in Figure 1. The eluate was evaporated with nitrogen to dryness and reconstituted with 50 µL of 20:80:1 (v:v:v) acetonitrile:water:ammonia.
An ACQUITY Premier UPLC I-Class FTN System was used to separate the Aβ peptide isoforms using a ACQUITY UPLC BEH Peptide C18 300 Å, 2.1 x 150 mm, 1.7 µm Column with a 0.3% NH4OH/water/acetonitrile gradient from 10–55% mobile phase B and analysed on a Xevo TQ Absolute Mass Spectrometer using the MRM transitions and precursor scan parameters listed in Table 1. Mobile phases were prepared freshly on daily basis.
Results and Discussion
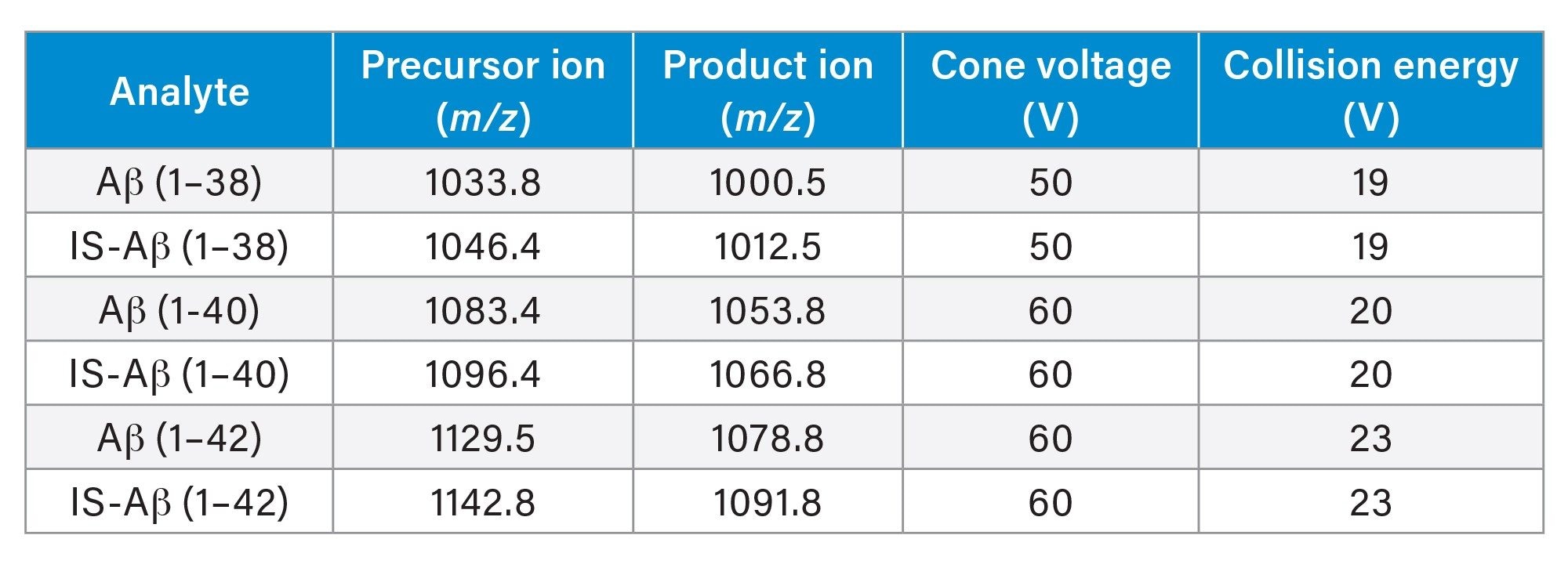
Analytical sensitivity of the lowest calibrator at 0.1 ng/mL was demonstrated with S/N (PtP) > 10:1 for all Aβ peptide isoforms (1–38, 1–40, 1–42) across the five analytical runs (Figure 2).
The calibration lines over five analytical runs were linear with r2 > 0.999 for each of the peptides over the range 0.1–10 ng/mL.
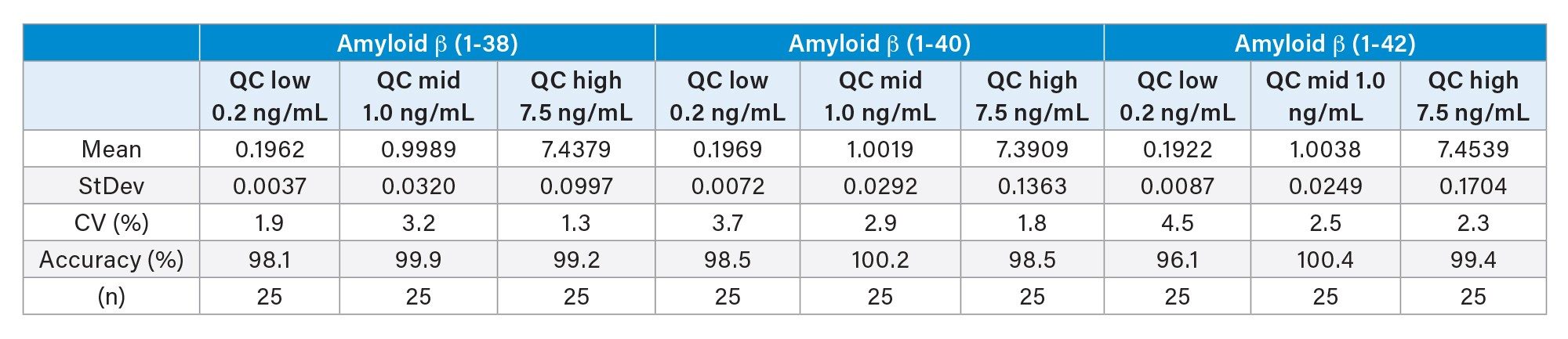
Total precision and repeatability across the Aβ peptides on the Xevo™ TQ Absolute Mass Spectrometer was evaluated using QCs at three concentrations (0.2, 1.0, and 7.5 ng/mL), in replicates of five over five analytical runs (n = 25). Total precision and repeatability were determined to be < 5% CV, the accuracy of the QCs compared to nominal concentrations ranged from 96.1–100.4% across the Aβ peptide isoforms (Table 2).
Conclusion
A LC-MS/MS method for the analysis of Aβ peptide biomarkers in artificial CSF was developed for clinical research. Through the use of the ACQUITY Premier UPLC I-Class System and Xevo TQ Absolute Mass Spectrometer excellent inter-day linearity, analytical sensitivity, precision, and accuracy can be achieved, providing confidence in the results obtained for quantification of Aβ peptide isoforms (1–38, 1–40, 1–42) in clinical research.
References
- Jensen M, Hartmann T, Engvall B, Wang R, Uljon SN, Sennvik K, Naslund J, Muehlhauser F, Nordstedt C, Beyreuther K et al: Quantification of Alzheimer Amyloid Beta Peptides Ending at Residues 40 and 42 by Novel ELISA Systems. Mol Med 2000, 6(4):291–302.
- Lachno DR, Evert BA, Maloney K, Willis BA, Talbot JA, Vandijck M, Dean RA: Validation and Clinical Utility of ELISA Methods for Quantification of Amyloid-beta of Peptides in Cerebrospinal Fluid Specimens from Alzheimer's Disease Studies. J Alzheimers Dis 2015, 45(2):527–542.
- Salcedo J, Ph. L, Davey L, Lame M, Dunning C, Chambers E: Amyloid Beta Peptides Quantification by SPE-LC-MS/MS With Automated Sample Preparation for Preclinical Research and Biomarker Discovery. Waters Application Note, 720006517.
720007900, March 2023